
Corrosion of metals-SUBSTANCES AND their PROPERTIES - LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to repeat and expand the idea of corrosion, its types, mechanisms; to consolidate knowledge of ways to protect corrosion metals.
Basic concepts: corrosion, chemical corrosion, electrochemical corrosion, oxidation, reduction processes, protector, passivation, inhibitor.
Equipment: Zn-wire, granule; Withu-wire; NSL-section. CuSO4.
Electrolytes NaOH (10%), H2O — dist., iron nails — 5; NaCl — p-p, K3[Fe(CN)6], iron, tinned or galvanized; 5 test tubes No. 1 - Fe, NaOH; No. 2 - Fe, NaCl; No. 3 - Fe, Cu, NaCl; No. 4 – Fe, Zn, NaCl; No. 5 — Fe, H2O; No. 1 — HCl, tinned iron, 1-2 drops To3[Fe(CN)6]; No. 2 - hcl, galvanized iron, 1 t. 2 K., K3[Fe(CN)6].
Lesson progress
I. Organizational moment
Results of independent work, analysis of mistakes made, explanation of the correctness of certain tasks.
II. Discussion of homework completion
№ 4 § 18
![]()
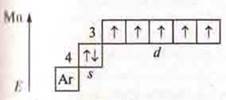 The
maximum C. O. is +7. Oxide
The
maximum C. O. is +7. Oxide![]() is an
acidic oxide, it corresponds to the acid Nmpo4.
is an
acidic oxide, it corresponds to the acid Nmpo4.
Metal element
![]()
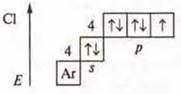 The
maximum C. O. is +7. Oxide
The
maximum C. O. is +7. Oxide![]() is an
acidic oxide, it corresponds to the acid HC-4.
is an
acidic oxide, it corresponds to the acid HC-4.
Non-metallic element
On the basis of the structure of the m atom, the n-metal element and the SL-nonmetallic atom and the maximum S. O. form acid oxides, which correspond to acids. In The Mendeleev PSC, Mn and SL are located in the same group VII, but in different subgroups. Cl — in the main subgroup, Mn — in the secondary subgroup.
№ 10 § 18
a) Be, Mg, CA; CA in all elements +2, because they are located in group II, the main subgroup, at the external energy level have two electrons. These are typical metals. The radius of the atoms of these elements is different. From Be toCA the radius of the atom increases, metallicity increases from Be toCA. They form oxides of BeO, MgO Cao, the nature of which varies from amphotericVEO to the main Cao. Hydroxides formed by these elements-bases, the nature of which varies from amphoteric — Ine(HE)2, showing acid-base character to the base with a pronounced property of a typical base;
b) Na, Mg, Al. The metals are located in the III period of the Mendeleev PSCE. From Na to Al, the charge of the nucleus increases, the number of electrons of the external energy level increases, the radius of atoms contracts — metallic properties weaken, non-metallic ones increase.
Na
is a metal, Mg is a metal, andl is a transition element. the CA increases from
+1 to +3. The elements Na, Mg, Al form oxides ![]() ,
,![]() the
character of which changes from basic to amphoteric, the PH increases, the
radius of ions decreases.
the
character of which changes from basic to amphoteric, the PH increases, the
radius of ions decreases.
These oxides correspond to hydroxides NaOH-alkali Mg(OH)2-bases, Andl(HE)3-amphoteric base, shows acid-base properties. Thus, by the end of the period, metallicity weakens, the basic character of the oxide weakens, the acidic character increases, through amphoteric. The character of hydroxides changes from the base, through amphotericity to acid due to a decrease in the radius of the atom, the radius of the ion and an increase in S. O.: there is an increase in the acidic character.
III. Learning new material
Plan of presentation
1. Work with the text of the textbook p. 208. Determination of metal corrosion.
2. General concepts of metal corrosion.
3. Types of corrosion: a) chemical corrosion; b) electrochemical corrosion, the influence of the medium on electrochemical corrosion.
4. Methods of protection against corrosion:
* passivation of metals;
· creating polished surfaces;
* electrochemical method of protection: a) protective: b) cathodic;
· environment processing: a) introduction of substances that slow down corrosion, corrosion inhibitors; b) removal of dissolved2Oxygen — deaeration.
5. The ability of metals to corrode (communication students).
The study of new material begins with reading the text of § 18 p. 208. The concept of "corrosion" is written in a notebook.
Corrosion is the process of spontaneous destruction of metals and alloys under the influence of the external environment (lat. corrosto — corroding).
All metals are reducing agents. The phenomenon of corrosion is a manifestation of the reducing properties of metals. Knowing the essence of this process will make it possible to protect the metal from corrosion. Substances that cause metal corrosion are oxidizing agents, such as non-metals (oxygen, etc.), water, inorganic and organic acids, oxides, and alkalis. They can be found to some extent in the environment — air, soil, water.
The oxidized form of metals is their oxides.
Example: As a result of iron corrosion, iron oxide (III). which is apart of rust — nFe2O3· mn2O. The concept of "rusting" characterizes specifically the corrosion of iron. Rust is porous, flakes off and does not protect the iron from further destruction. Due to corrosion, a huge amount of iron is destroyed. In the XIX century, half of the iron smelted was destroyed, because there were no known reliable methods of fighting corrosion. Currently, 1/6 of the molten cast iron turns to rust. The following conclusion is drawn from the above: the fight against corrosion of iron and its alloys is the most important task facing humanity.
Question: In the capital of India, Delhi, there is a shesgitonnaya iron column, made in the beginning of the fifth century. It is not subject to corrosion, even in spite of the humid and warm climate of India, which should favor the flow of corrosion processes. Why?
Answer: This column is almost 99.8% made of iron. It is still not clear how the Indian craftsmen managed to obtain pure iron in ancient times.
Modern industry and technology operates metals and their alloys often at high temperatures, high pressures, in aggressive environments — acids, alkalis, salts; and the air is also clean, especially in large cities. The fight to preserve metal from destruction and corrosion is one of the most important tasks of our time.
To better protect the metal from corrosion, it is necessary to understand its chemical nature.
Corrosion is окислительноa redox process that occurs in different environments. Dry medium — gas corrosion, medium - liquid corrosion. The liquid medium can be represented by electrolytes or non-electrolytes. There is thus a distinction between chemical and electrochemical corrosion.
In chemical corrosion, there is a direct interaction of metal with an oxidizer, and the transition of electrons from the metal atom to particles, atoms, and molecules of the medium is observed. Industrial emissions, such as SO2— sulfur oxide (IV), are often present in the atmosphere.
Chemical corrosion is possible according to the equation

In the composition of natural gas, there may be an impurity-hydrogen sulfide, which reacts with a metal pipe through which the gas is transported. The nozzle of the rocket engines interacts with the oxidizer of the fuel. Details of oil-producing structures interact with oil and its processed products.
However, in natural conditions, corrosion mainly occurs in a different type. As a rule? metal products come into contact with electrolytes — aqueous solutions of acids, alkali salts, that is, with a conductive medium.
Such corrosion is called electrochemical. Water is a weak electrolyte that is often present as a film on the metal surface. This film dissolves atmospheric gases — oxygen, carbon dioxide, impurities in the form of SO2-sulfur oxide (IV), NO2 -nitrogen oxide(IV), etc., resulting in an acidic layer. It should be known that the contact of metals can be directly with electrolytes. When storing metals in atmospheric conditions, electrolytes can be formed immediately if water or moist air enters the container. This is typical of large cities and industrial centers.
Most of the metal structures — bridge supports, oil rigs, a group of river and sea transport — are directly in contact with river, lake, sea water, where salts are dissolved, in contact with the soil, where salts are also present.
Electrochemical corrosion is significantly different from chemical corrosion (show the experiment with the nail). In the aquatic environment, there is an oxidizer-oxygen dissolved in it and a small amount of hydrogen cations, which are formed when the pod dissociates. It is possible to recover cation of hydrogen:
![]()
and recovery of oxygen:
![]()
For these processes, electrons are represented as metal atoms, since they are reducing agents:
![]()
The predominance of a particular recovery process depends on the environment. In an acidic environment, the process of reducing hydrogen prevails, the hydrogen cation H+is the metal oxidizer. If the medium is alkaline, neutral-the oxidizer is oxygen.
As a result of electrochemical corrosion, there is always an electric current. The current strength will depend on what impurities are present in the metals, as well as which metals are in contact.
Experience.
1. Compare the reaction rates of two experiments (codoscope).
a) the interaction of zinc with a solution of hydrochloric acid. The release of hydrogen is observed:
![]()
b) touch the zinc granule with a copper wire. There is a very rapid release of hydrogen. The speed of reaction increased.
2. Compare the reaction rate of zinc with hydrochloric acid at first, and then add a few drops of CuSO4 solution. The speed of reaction increased. Why does this happen?
1.
Zinc is a more active metal than copper. When the zinc granules come into
contact with a copper wire the electrons from the more active metal Zn pass to
the less active metal copper: ![]() a
layer of zinc cations is formed on the zinc border. This is the positive layer,
and the copper wire gets a negative charge, becomes a cathode, where hydrogen
cations begin to discharge very quickly:
a
layer of zinc cations is formed on the zinc border. This is the positive layer,
and the copper wire gets a negative charge, becomes a cathode, where hydrogen
cations begin to discharge very quickly: ![]()
The reaction equation in General looks like this:
![]()
There is a directed movement of electrons — an electric current occurs.
In the second experiment, when adding the CuSO4 solution, the following occurs. Zinc is more active than copper, so it begins to displace it from the salt solution. Copper settles on the zinc granule, and then, as in the first experiment, the areas of pure copper are charged negatively, becoming a cathode, where hydrogen atoms are quickly discharged.
Next, we need to consider Fig. 41 of the textbook on page 211 "the process of destruction of an iron sample in the presence of copper impurity in an acidic, alkaline and neutral medium" in comparison with the experiments carried out. Make notes in your notebook.
2.
Iron is more active than copper, there is a recoil of electrons, the iron atom
acquires the charge of the particle 2+, becomes an anode![]() Electrons
move directionally to the impurity particle from copper, so that it acquires a
negative charge — it becomes a cathode. If this particle-impurity has contact
with the electrolyte medium, where hydrogen cations are present, their
discharge occurs
Electrons
move directionally to the impurity particle from copper, so that it acquires a
negative charge — it becomes a cathode. If this particle-impurity has contact
with the electrolyte medium, where hydrogen cations are present, their
discharge occurs![]() withummarno
the equation of the process looks like this:
withummarno
the equation of the process looks like this:
![]()
In
the case of alkaline and neutral environments, the process of discharging iron
atoms occurs in the same way:![]() However,
an impurity particle from the copper cathode will discharge oxygen with the
formation of hydroxide ions:
However,
an impurity particle from the copper cathode will discharge oxygen with the
formation of hydroxide ions: ![]() then
iron cations and hydroxide anions form iron (II) hydroxide:
then
iron cations and hydroxide anions form iron (II) hydroxide:![]() which
in the presence of oxygen and water passes into iron (III):
which
in the presence of oxygen and water passes into iron (III):
![]()
In the future, the decomposition of iron (III) hydroxyl when heated — solar energy — forms iron (III) oxide Fe2O3, which is part of the rust.
IV. Generalizations and conclusions
1. Corrosion is окислительноa redox process.
2. Corrosion is chemical and electrochemical.
3. In the case of galvanic corrosion is always formed by an electric current. There is a breakdown of the more active metal, which acts as an anode, and the less active metal — as a cathode.
4. the rate of corrosion depends on the reducing capacity of the contacting metals. The more they differ in their regenerative capacity, the greater the reaction rate.
Next, students are offered a small message about the ability of metals to corrode.
Vse typical metals in the main subgroups I and II of D. I. Mendeleev's PSC groups have low corrosion resistance. Metals of the secondary subgroup of group I are resistant. This is Withu, Ag, AI. As the element's ordinal number increases, it increases.
Metals of the secondary subgroup of group II are more stable, because on their surface, under the action of external oxygen, strong oxide films are formed that protect them from the environment.
Metals of group III, the main subgroup. For example, Al is protected by an oxide film, which has high protective properties, but can be destroyed in acids and alkalis.Al is passivated from the surface of concentrated H2SO4and HNO3.
Metals of group IV, the main subgroup of Sn and Pb are resistant to corrosion and are coated with a strong oxide film.
Metals of the V, VI, VII, VIII groups of secondary subgroups are capable of passivation, have a high corrosion resistance. Metals of group VIII, a secondary subgroup of Os, Ir, and Pt, have the highest resistance. Iron is passivated in the cold with concentrated nitric and sulfuric acids from the surface.
On totransparency possible to show the following table.
|
Signs |
I |
II |
III |
IV |
V |
VI |
VII |
VIII |
the larger the serial number, the higher the anticorrosive resistance |
||||
|
of the GL. |
+ persons. Me |
CH. |
POB. |
CH. Al |
POB. |
CH. |
POB. |
POB. |
POB. |
POB. |
POB. |
||
|
High corrosion resistance |
+ |
+ |
+ |
+ |
+ |
+ |
|||||||
|
Low corrosion resistance |
+ |
+ |
|||||||||||
|
Passivated in HNO3(K) and H2SO4(K) |
+ |
+ |
+ |
+ |
|||||||||
|
Covered with a durable black oxide coating |
+ |
+ |
+ |
+ |
+ |
+ |
|||||||
Thus, knowing all types of corrosion and chemical processes occurring during corrosion, you can offer the following methods of protecting metals from corrosion.
1. Isolation of metals from the external environment:
a) non-metallic coatings of metals with varnish, paint, enamel, resin, oil, polymers;
b) metal coating of metals — zinc plating, aluminumplating , copper plating, chrome plating , Nickel plating, gilding, silvering. All these coatings create a decorative fork for the products. The metal is zinc-plated if the medium is aqueous; in the case of operations in sulfuric acid, it is coated with lead, since lead is passivated with sulfuric acid to form a PbSO4 layer4.
Tinning of metal-coating of kitchen utensils with tin. White tin is used for canning.
Experience.
Metal coatings are protected from the action of hydrochloric acid by rubber, phenol-formaldehyde resin. For car parts, anti-corrosion coatings in the form of rubber , epoxy, bituminous mastic are used.
In
two solutions of hydrochloric acid, add salt K3[Fe(CN)6].
In one solution, lower a scratched plate of tinplate (iron coated with tin), in
the other-a scratched galvanized plate (iron coated with zinc). The result of
the observation is as follows: in the first solution, there is a color change,
because tin is less active than iron, and in the solution, iron will first be
destroyed: ![]()
![]() a
solution of K3[Fe(CN)6] — a reagent in the presence of Fe2+gives
a dark blue coloration (turbulence blue). In the case of a galvanized plate,
the more active metal-zinc-will first be destroyed:
a
solution of K3[Fe(CN)6] — a reagent in the presence of Fe2+gives
a dark blue coloration (turbulence blue). In the case of a galvanized plate,
the more active metal-zinc-will first be destroyed: ![]() and the
staining of the solution does not occur.
and the
staining of the solution does not occur.
C) carry out processing of the medium:
heating water-when heated, the solubility of gases decreases;
the water passing through the iron turnings — removal of oxygen deaeration;
adding sodium sulfite to water — chemical removal of oxygen:
![]()
d) carry out chemical coating: oxidation, nitriding, carburizing of the surface, introduction of substances that slow down corrosion-inhibitors.
e) for the destruction of scale-rust on the weapon metal, "pickling soups" are used — a solution of H2SO4 with the addition of brewer's yeast, starch, flour. When transporting hydrochloric acid, butylamines are used. More than 5 thousand inhibitors are known: amines, nitrites of amines, esters.
2. Creation of polished surfaces, so that moisture does not linger, better formed oxide film, because the surface layer becomes homogeneous.
Passivation of metals-production of alloys with the addition of me- / ii.iюн yn Cr, Ni, AI, Mn, Mo, W, V having protective oxide films.
3. Electrochemical methods of protection:
a) cathodic — anodic protection. A more active metal (the anode) is attached to the metal structure. In this way , ship hulls , oil towers, and pipelines are cleaned. Mg, Al , Zn are used as the active metal, Zn;
b) cathode protection — a metal structure is attached to the cathode of an external source so as to exclude the possibility of its anode destruction.
Experience.
The experiment on electrochemical corrosion of iron should be prepared a few days before the lesson.
5 vials:
No. 1: Fe, NaOH; No. 2: Fe, NaCl; No. 3:Fe, Cu; No. 4: Fe, Zn, NaCl; No. 5 — Fe, H2O-boiled.
Iron nails in test tube No. 3 are connected with copper wire; in test tube 4-with zinc wire.
After adding all test tubes 1-2 K. K3[Fe(CN)6] color change to blue, marked by the intensity of changes in the cyst and the rate of corrosion.
V. Homework assignment
§ 18 p. 208-214. P. 223. № 17, 18. 19 (orally).
VI. Pinning
№ 14, 15. 16 § 18.
Answer:
No. 14 § 18. Corrosion — spontaneous destruction of metals and alloys under the influence of the external environment. Corrosion can be chemical or electrochemical. Electrochemical corrosion is a physical and chemical process.
No. 15 § 18. a) it is chemical corrosion: b) this is electrochemical corrosion — destruction in a current-conducting medium.
No. 16. a) if tin is coated: pH > 7, when damaged, iron — a more active metal-will be destroyed.
![]()
![]()
b) in an acidic environment, pH < 7
![]()
If coated with zinc: at pH ≥ 7, pH < 7.
When damaged, the zinc will break down as the more active material.
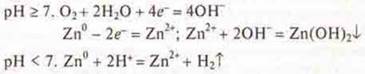
Answers to homework
§ 18 № 18.
a) Yes, magnesium is more active than iron and will break down first;
b) no, Pb is less active than iron; iron will break down first;
C) Ni-Nickel is less active than iron, but is covered with a protective, strong oxide film. It does not break down and does not allow Nickel to break down. The iron will be protected.
§ 18 № 19. Paint "Serebryanka" or "serebrin" in the composition contains aluminum powder. Al is coated with a very strong anti-corrosion oxide film. Al does not corrode and the surface of tanks for a certain time (until it "deteriorates" — dries) and thanks to vegetable oil is protected from the external environment.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.