
Integrated lesson on the theme "Metals in nature. Methods of obtaining metals. Alloys" - SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
The subject of the lesson includes material that is studied in the course of geography, physics, biology, ecology and chemistry; teachers of the above-mentioned subjects participate in this lesson.
The purpose of the lesson: the geography teacher explains to students the concept of "ore"; introduces a collection of ores; finds out if they meet in the region of residence, laboratory experience learn to identify jeleznyaki, work with map of region of residence;
the chemistry teacher gives the concept of "metallurgy"; tells about the development of the metallurgical industry of the region of residence, introduces the ores of metals that occur in nature, analyzes the main methods of obtaining metals: pyrometallurgy hydrometallurgy, electrometallurgy; experimentally examines pyrometallurgy, hydrometallurgy: strengthens the ability to make OVR; gives a General idea of alloys; conducts laboratory experience-introduces metal alloys; gives an idea of the use of alloys in various areas of human life;
the physics teacher gives an idea of the electric current in solutions and melts of electrolytes; introduces students to Faraday's laws, teaches them to solve calculation problems based on Faraday's laws, and experimentally conducts electrolysis of a solution of copper sulfate (II);
biology teacher talks about the use of alloys in medicine;
the teacher of ecology-about the protection of the environment, prepares essays with students-messages.
Basic concepts: ore, metallurgy, pyrometallurgy, hydrometallurgy, electrometallurgy, electrolysis, anode, cathode, oxidation, reduction, oxidizer, reducing agent, electroplating, electroplating, alloy.
Equipment: on the students ' desks for laboratory experience of metal ores:
oxides: Fe3About4, Fe2O3, NH2O, Al2O3, CuSO4, Fe (paper clip);
sulfides: Cu2S, PbS, FeS2, KCl, NaCl, (CuOH)2CO3, CuSO4· H2O, Na3AlF6; mica, feldspar. Map of the region of residence, compass, collection "Metals and alloys".
On the teacher's Desk CuO, HCl(K), Ag2O (NH3· H2O) glucose; rectifier, CuSO4, electrolysis device, metal tripods, test tubes, holder for the device, alcohol lamp; codoscope, codotransporants; thematic Newspapers and scientific and educational literature, abstracts.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson. Organization of students for active work and during two lessons; instruction on the preparation of a reference summary.
Recommendations for the teacher
1. when conducting an integrated lesson, students make a summary of the lesson in the form of a plan (based on the presentation plan given in the book).
The content of this lesson can be shortened for a regular combined lesson of 45 minutes instead of an integrated one.
Plan of presentation
1. The combination of metals in nature (chemistry teacher).
2. Ore metals. Finding metal ores on the territory of the region of residence. Metallurgy. Development of metallurgy in the region of residence. Conducting laboratory experiment # 1. Acquaintance with metal ores and determination of iron ores (teachers of geography and chemistry).
3. Methods of obtaining metals:
a) pyrometallurgy (experiment), production of copper, silver:
b) hydrometallurgy (experimental production Withu. Conducting laboratory experiment # 2). Obtaining copper by substitution reaction.
4. Electrometallurgy (chemistry teacher). Fundamentals of the theory of electrolysis. Electric current in solutions and melts of electrolytes.
Solution of calculation problems based on Faraday's laws and electrolysis equations (experiment: electrolysis of copper sulfate solution (II)) physics teacher).
5. production of aluminum by electrolysis of aluminum oxide melt. Electrolysis of molten salts of active metals (chemistry teacher).
6. Application of electrolysis of solutions and melts of salts (physics teacher).
7. Environmental protection: air, soil, water (communications 1-2 students).
8. Metals in pure form, metal alloys and their application. Conducting laboratory experiment # 3. Familiarization with metals and alloys. Working with the collection (chemistry teacher).
9. The use of alloys in medicine (biology teacher).
10. Generalization and conclusions on the key issues of the topic.
11. Homework assignments
§ 18 p. 214-223, № 25, 27.
Task. Determine the volume of gas released at the anode during the electrolysisof a CISO 4 solutionweighing 320 g, WCuS04= 20%.
III. Learning new material
1. Prepare the electrolysis of the melt solution of vasl2.
2. Solution of the problem of electrolysis by the equations of electrolysis of solutions and melts of salts.
Task. During electrolysis of a 7.7 g sodium chloride melt, gas was released at the anode. Determine the volume of released gas.
The lesson begins with the chemistry teacher talking about the following questions:
Question: What is the conclusion about the activity of metals based on their chemical properties?
Answer: According to the activity of metals can be divided into a) very active, in the electrochemical series of voltages are conventionally located from Li to Al; b) metals of medium activity from Al— Pb; C) metals of low activity from Cu—Au.
Question: In what form can metals be found in nature?
Answer: You can find metals in the form of compounds, and low-active ones in free pure form.
Question: What kind of compounds do you think you can find in the form of active metals, metals of medium activity, and low-activity metals?
Answer: These can be salts or oxides. Very active metals in nature occur in the form of salts: chlorides, nitrates, sulfates, phosphates, carbonates; aluminum — in the form of aluminum oxide. The example on totransparency:
chlorides: NaCl, KCl;
sulphates: CaSO4· 2H2O-gypsum, Na2SO4· 10H2O-Glauber's salt;
nitrates: NaNO3-Chilean nitrate, Ca(NO3)2-Norwegian Pamir;
phosphates: CA3(PO4)2-phosphorites; carbonates: SASO3-chalk, marble, limestone;
MgCO3-magnesite, SASO3· MgCO3-dolomite, (WithuON)2CO3-basic salt, malachite;
silicates — 2-aluminosilicates, feldspar, mica, kaolinite-they contain aluminum.
Metals of medium activity are found in the form of oxides and sulfides, iron Oxides: Fe2O3— red ironstone, Fe2O3· NH2O — brown ironstone, Fe3O4-magnetic ironstone.
Sulfides: Cu2S — copper luster, PbS-lead luster, ZnS — zinc bling, FeS2-pyrite, HgS-cinnabar.
Metals of low activity are found:
a) in free form-Au and Pt;
b) in free form and in the form of compounds: Ag, Hg, Cu, Sn.
Question: What are all these compounds called? What common name can I give them?
Answer: These are metal ores.
Then the lesson is taught by a geography teacher.
Ores are minerals and rocks that contain metals or their compounds, and are suitable for industrial production of metals. If the ore consists of two or more metals or their compounds, the ores are called polymetallic (ferrous chromium, lead-silver ores, copper-molybdenum ores).
The teacher organizes students for laboratory work No. 1:
a) acquaintance with metal ores: in the form of salts — sodium and potassium chloride, sulfide ores, bauxite ores. Note their appearance, possible similarities:
b) the teacher pays attention to ores containing iron. This is the ironstone: red, brown, magnetic. These are oxide ores.
Students in notebooks, and the teacher on the blackboard make a table and under the guidance of the teacher describe their observations.
|
Ore |
Color |
Trait on porcelain |
Magnetic properties (compass, magnet) |
|
Fe3O4-magnetic ironstone |
Dark grey |
Black |
It has magnetic properties |
|
Fe3O3— red ironstone |
Red |
Red-brown |
weak Magnetic properties |
|
Fe3O3· nH2O-brown ironstone |
Brown |
Brown |
Does not have magnetic properties |
These ores — oxide-are the most suitable for obtaining metals.
Then the teacher suggests that students work with the map of Russia and the map of the region of residence. Find the deposits of these ores and draw conclusions.
The industry that is engaged in the production of metals from ores is called metallurgical. The science of industrial methods of obtaining metals from ores is called metallurgy. The production of iron and its alloys is considered to be ferrous metallurgy. The production of other metals is related to non-ferrous metallurgy.
Then the explanation goes to the chemistry teacher.
And what if the ore is not oxide, but in the form of salts of chlorides, sulfates, and sulfides? That's why we need to know how to get from these compounds to the pure metal. That is, methods of obtaining metals.
The first method is pyrometallurgy. This is the reduction of metals from ores at very high temperatures using carbon, carbon monoxide (II), hydrogen, metals — aluminum, magnesium, calcium.
Example: reducing copper from cuprite by calcining it with coke:
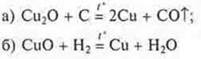 - the
experiment is demonstrated by the teacher.
- the
experiment is demonstrated by the teacher.
Experiment. Over a hot powder WithuO hydrogen is passed. Bright copper sheen on the test tube walls and water droplets are the result of a reaction.
Experiment. The "silver mirror" reaction in organic chemistry:
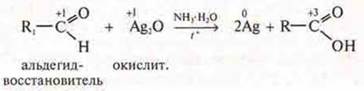
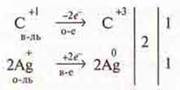 There
is a silver Deposit on the walls of the test tube.
There
is a silver Deposit on the walls of the test tube.
However, copper, lead, and zinc occur naturally as sulfides. Sulfide ores are first fired to metal oxide, and then the resulting oxide is reduced by coal.
The teacher calls the student to the blackboard, and the student composes chemical reactions, treating them like IBS.
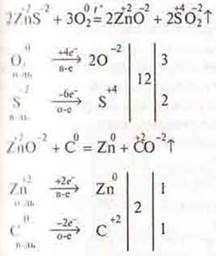
If the ores are carbonate, then they are immediately calcined with coal, because carbonates decompose at high temperature to metal oxides andCO2. In the case of malachite (WithuHE)2CO3.
Experiment:
![]()
In industry, stranding from copper(II) oxide is reduced with coal:
![]()
as well as Fe, Cd, Zn, Je, Sn, Pb metals. These metals do not form carbides with coal.
We are convinced of the good qualities of hydrogen as a reducing agent. In modern metallurgy, this reducing agent has become more frequently used. The metal is obtained as a powder. Metallurgy is called powder metallurgy.
![]() — moderadamente.
— moderadamente.
Active metals are also used for recovery — this is metallothermy:
![]() —
aluminothermy;
—
aluminothermy;
![]() —
magniture.
—
magniture.
Question: What is the process that occurs in all the disassembled examples when obtaining pure metal? Write it down in General terms:
![]()
There is a process of reduction — the acceptance of electrons, metal cation-oxidizer, CP increases.
The next method of obtaining metals is hydrometallurgy. This is the reduction of metals from solutions of their salts more active by metal. The process takes place in two stages:
Stage I. Dissolving a natural compound in a suitable reagent to produce a salt solution;
Stage II. From the resulting salt solution, the metal is displaced by a more active metal.
Experiment and laboratory experience 2.
The teacher dissolves the resulting copper(II) oxide and malachite in sulfuric acid or a ready-made chemical reagent WithuOh, or again calcining malachite in the beginning and getting SIO.
The teacher conducts the experiment according to the first stage.
![]() —
solution of copper (II) sulfate.
—
solution of copper (II) sulfate.
At the second stage, students conduct a laboratory experience 2.
Aniron clip is added to the CuSO 4 solution(it is available on tables) :
![]()
and make up the IAB.
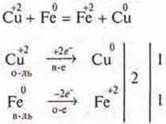
This method is somewhat expensive and lengthy. Why? For these steps, it is necessary to obtain salt by consuming ready-made reagents, and to obtain metal, an active, pure metal is needed. It must also be received. For the production of very active, medium activity and low — activity metals, electrometallurgy is used — it is the reduction of metals in the process of electrolysis of melts or solutions of salts. This question is very closely related to physics. To explain the theory in more depth, we need a physics teacher.
The physics teacher tells the story of an electric current in solutions and melts of electrolytes.
Liquids, like solids, can be dielectrics, semiconductors, and conductors:
dielectrics-liquid, water;
conductors — solutions and melts of electrolytes (acids, alkalis, salts);
semiconductors the melt of the sulfides, the molten selenium.
When electrolytes are dissolved in the will, an electrolytic dissociation occurs электролититическая диссоциаиия. Charge carriers in aqueous solutions or melts of electrolytes are anions ( - ) and cations (+).
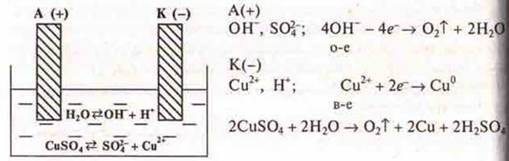
If the vessel with the electrolyte solution is included in the electrical circuit, the anions (-) will begin to move to the anode (+), and the cations (+) — to the cathode (-). As a result, an electric current will be established — the directed movement of charged particles. This conductivity is called ionic.
With ionic conductivity, the origin of the current is associated with the transfer of matter. On the electrodes, substances that are part of the electrolytes are released. The process of separating substances at the electrode, associated with redoxreactions, is called electrolysis.
Experiment:
Electrolysis of the copper(II) sulfate solution is performed and the chemistry teacher explains the OVR of electrolysis.
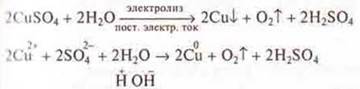
 According
to a number of stresses, the copper cation has a greater oxidizing ability.
Copper is restored at the cathode!
According
to a number of stresses, the copper cation has a greater oxidizing ability.
Copper is restored at the cathode!
![]()
There is a water discharge on the anode, but students can be explained as follows:
Water is a weak electrolyte, but the anode also has dissociation. We will record the discharge of an anion, which is simpler in composition, i.e. ITis -:
![]()
Oxygen is released at the anode.
Thus, to find out which cation is being discharged, you should look at its position in the electrochemical voltage series.
Example: a) there is a mixture of salts in the solution and cations are present: Cu2+, Ag+— Ni2+ - the discharge goes in the sequence: Ag+, Cu2+, Ni2+;
b) the solution contains cations Ni2+, H+.
If the concentration of Ni2+is very high, then they are discharged first, with a decrease in the concentration of Ni2+, there is a discharge of H+and Ni2+.
To find out the discharge of anions there is a genus of anions in descending order of their reducing capacity:
![]()
Decreases the ability to oxidize (discharge at the anode)
Students in the course of the experiment (at the end) are shown an electrode with copper and isolated bubbles of About2 on the anode. The codotransporant shows a drawing of the electrolysis of the CuSO4 solution.
Electrolysis of the NaCl melt:
![]()
Molten aluminium oxide in cryolite:
![]()
The physics teacher then explains Faraday's laws.
The dependence, called Faraday's law, was first established experimentally by M. Faraday in the 1930s. This law determines what determines the mass of the substance released on the electrode for a certain time. Find out it.
It is obvious that m = m0· N, where m0is the mass of one ion; N is the number of ions.
![]()
where M is the molar mass, Nais the Avogadro number.
On the other hand, the number of ions deposited on the electrode can be found as follows
![]()
where
Q is the charge passed through the electrolyte; q0is the charge of
one ion. So,![]() the
charge of the ion q0= e-· Z (the charge of the electron
multiplied by the valence).
the
charge of the ion q0= e-· Z (the charge of the electron
multiplied by the valence).
Thus,
![]()
NAand
e- - const. M and Z are constant for a given substance. So, we can
designate ![]()
Then
Faraday's law is written simply: ![]()
where K is the electrical equivalent (kg/KL).
Faraday's law: the mass of the substance released at the electrode is proportional to the charge passed through the solution.
The mass of the substance released at the electrode is proportional to the current strength and time.
Physical meaning of K: the electrochemical equivalent is equal to the ratio of the mass of an ion to its charge:
![]()
Based on Faraday's law, the value of the elementary electric charge e-= 1.6 * 10 -19 KL was obtained in 1784-19.
The chemistry teacher explains further the electrolysis of molten salts, oxides to produce very active metals.
Example. Electrolysis of the NaCl melt:
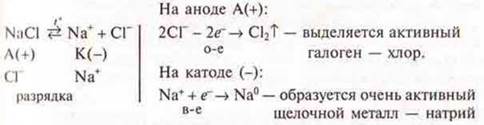
Aluminum production is very energy intensive process, for the production of 1 ton of aluminium you need 19 000 kWhsince t° melting of Al2O3of more than 2000°, to reduce this t° melting lead electrolysis in cryolite, Na3AlF6And(+) — carbon rods, (+) — the bottom of the bath.
![]()
The current is very high (several tens of thousands of amperes), the heat generated by the current keeps Al in a molten state.
Writing equations of electrolysis and the knowledge of Faraday's law was also necessary to solve computational problems.
The physics teacher gives an algorithm for solving computational problems according to Faraday's law.
Task 1
At a current of 1.6 A, 0.316 g of copper was deposited on the cathode of the electrolytic bath in 10 minutes. Determine the electrochemical equivalent.
![]()
Answer: K = 0.33 · 10-6kg/CL.
Task 2
When Nickel plating the part for 2 hours, the current passing through the bath was 0.25 A. K = 3 * 10-7kg/CL, s = 8,9 · 103kg /m3.
What is the thickness of the Nickel released on the part 0.2 m2?
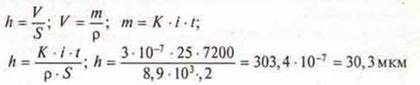
Answer: 7 = 30.3 µm.
The chemistry teacher gives algorithms for solving calculation problems both according to Faraday's law and the electrolysis equation.
It is experimentally established that when 96500 coulons are passed through the electrolyte solution, the equivalent of 96 500 electrolysis product is released — the Faraday number — F.
![]() Me-
the molecular weight of the element, n — the S. O. of the element.
Me-
the molecular weight of the element, n — the S. O. of the element.
![]()
Task 1
Calculate the mass of silver released at the cathode during electrolysis3of AgNO3 for 10 min. At a current of 5 A.
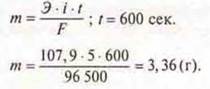
Answer: mAg= 3.36 g.
Task 2
Через
раствор ![]() пропущено
10 a of electricity is passed through the solution. 11.86 g of copper was
released at the cathode. Determine the time of electrolysis.
пропущено
10 a of electricity is passed through the solution. 11.86 g of copper was
released at the cathode. Determine the time of electrolysis.
![]() andwhether
10 min.
andwhether
10 min.
Answer: t = 10 (min).
Task 3
Calculate the mass of the released copper during the electrolysis of a solution of CuSO4weighing 160 g, WCuSo4= 20%.
1. Calculate the electrolysis equation:
![]()
2. Then everything is solved by the algorithm of solving problems, if the chemical equation is known:
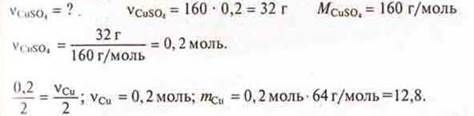
Answer: mWithu= 12.8 g.
IV. Messages to students
Applications of electrolysis:
1. Production of very active alkaline and alkaline earth metals by electrolysis of molten ores.
At present, the cost of production of aluminum is slightly reduced, because it is obtained from a melt of bauxite in cryolite and along with iron, it has become the most common in engineering and in everyday life.
2. Electroplating — coating a metal surface with a thin layer of difficultly oxidizable metals. Chrome plating, copper plating, Nickel plating, gilding, silvering. The cathode is the product, the anode is the metal that covers the product.
3. Electro — production of relief copies of metal objects. This method was invented by B. S. Jacobi in the 40s of the XIX century.and applied it to the production of hollow figures for the St. Isaac's Cathedral in St. Petersburg. Cast-cathode, metal-anode. Making copies in book printing. This is how they get stereotypes, only for books, of high-quality printing.
4. Cleaning of metals from impurities. Example: copper smelted from an ore is placed in a bath as an anode. During electrolysis, the copper of the anode dissolves, falls to the bottom, and pure copper is released at the cathode.
The chemistry teacher complements this point and explains to students the concepts of "soluble anode"; "copper block", " cathode (inert carbon)»:
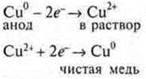
In its pure form, metals are not used in large quantities. Many of their physical properties are not suitable for products. Gold and aluminum are very malleable metals, chromium is very brittle, and so on. Therefore, they began to produce metal alloys.
In nature, an example of alloys: feldspar, mica, basalt and even glass. However, metallurgical alloys have a metallic chemical bond and have the same physical properties as the metals in the alloy, but slightly improved. Types of alloys (totransport):
a) solid solutions (atomic lattices of metals of the same type), and in the alloy crystal there may be different atoms:
Ag-Withu; Ag-AI; si — Ni;
b) just alloys (atomic lattices of different types, a mixture of individual crystals):
Pb — Sn, Bi — Cd, Pb — Ag;
C) intermetallic compounds (chemical compounds):
Zn and Withu; CA and Sb; Pb and Na.
Experience 3.
Students are introduced to the collection "Metals and alloys". The composition of some alloys is recorded in a notebook (codotransporant).
1)
Bronze  Machine
parts, artistic casting.
Machine
parts, artistic casting.
2)
Brass![]() Appliances,
machine parts, household appliances.
Appliances,
machine parts, household appliances.
3)
Duralumin ![]() Equal
to steel in strength, lighter by 3 times. In aircraft construction.
Equal
to steel in strength, lighter by 3 times. In aircraft construction.
4) low-Melting alloy.
Solders, "Tratnik" 1/3 Rb and 2/3 Sn.
5) Nichrome: Ni-67.5%; Withr-15%; Fe-16%; MP-1.5%. High-temperature. Heating device. Stainless steel, medical instruments, prosthetics.
6) Wins: C, W, СоIt is close to a diamond.
7) Babbit: Sn, Pb, Sb, Withu-fill bearings.
8) Sn 50%, In-50% - for bonding glass and metal.
Then the student's report is listened to, related to the requirements for the protection of soils, air, water in the production of metals, since there may be emissions of SO2, CO2 gases, ore particles into the atmosphere, which causes "acid rains", the death of plants, animals; human disease.
The biology teacher talks about the use of alloys in medicine.
Wars in the ancient world led to the fact that many soldiers were увеченыхmaimed and crippled. And even then there was the problem of replacing body parts with artificialones . The most successful were wooden leg prostheses (from the Greek. "protates" — joining), for the beauty wrapped in thin bronze sheet. Prosthetics became possible due to the fact that bone tissue can be replaced in nature. For example, dentures began to be made 3,000 years ago by the Etruscans — a people who lived in what is now Italy and were destroyed by the Roman Empire.
In the age of Enlightenment, attention was paid to beauty, a white smile. Prosthetics of teeth has become popular. They used wax, gold, and lead for prosthetics. Missing teeth were even replaced with wooden teeth. Modern dentistry uses various materials for prosthetics, including metals. Only mandatory knowledge about their compatibility and the oral cavity, where the environment is alkaline, and different metals in the electrolyte environment create a galvanic cell with a certain current strength, which is not safe for the body!
Modern prostheses are made of light metals and alloys, as well as polymers. They can bend in the joints, do not break down in the body's electrolyte environment. From metal alloys, rods are made to strengthen the bone in the case of severe fractures.
It will take a lot of knowledge for prosthetics to be able to replace limbs, so that a person can control them like their own hands and feet. These are the tasks of the future and they are for you, our younger generation, to solve.
V. Summarizing the lesson results
The chemistry teacher summarizes the lesson on nodal issues, sets a school grade, and sets homework.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.