
Non-metals-SUBSTANCES AND their PROPERTIES - LESSON PLANS for CHEMISTRY 11 class - lesson plans-lesson plans-author's lessons-plan-lesson summary - chemistry
Lesson objectives: to summarize information about the specifics of the structure of atoms of non-metals; learn to identify oxidation States; to consolidate knowledge of the characteristics of the structure, physical and chemical yourSTV PRostich substances — metals; to consolidate the ability to make the chemical reactions characterizing the properties of nonmetals.
Basic concepts: nonmetallicity, covalent bond, atomic and molecular crystal lattice, oxidizer, reducing agent, oxidation, reduction; electronegativity, allotropy.
Equipment: tables "Chemical bond", "Crystal lattices"; models of crystal lattices; nonmetals: S, Si; P (red); Br2; Ki; Zn, HCl, CIO; device for gas production.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson.
II. Checking that homework has been completed correctly
The teacher focuses the attention of students on the conditions of tasks in which different types are proposed: tasks for "excess" and "lack" - № 28, 30; tasks for mixtures - № 31, 32.
In order to save time lesson on totransparency to suggest that students verify the solution of task No. 28, 29, 30. Of challenge mixture: No. 31 and 32 — the teacher explains to students.
Task # 28
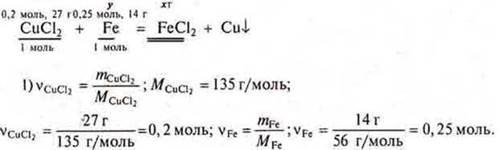
![]() a
mole of iron is required, and 0.25 mol ⇒
of Fe is given in excess. CCl2-a drawback, we calculate it based on
the drawback.
a
mole of iron is required, and 0.25 mol ⇒
of Fe is given in excess. CCl2-a drawback, we calculate it based on
the drawback.
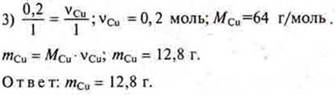
Task # 29
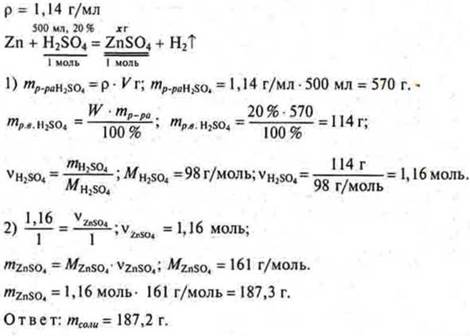
Task # 30

Task # 31.
Brief condition:
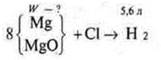
Decision:
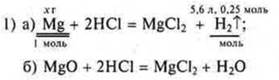
From
the equation "and» ![]()
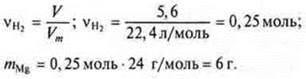
Since the reaction "b" does not give hydrogen, we immediately determine WMgin the mixture
![]()
Answer: WMgin the mix= 75 %.
Task # 32.
Brief condition:
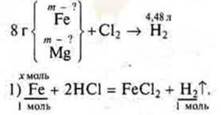 Take
Take ![]()
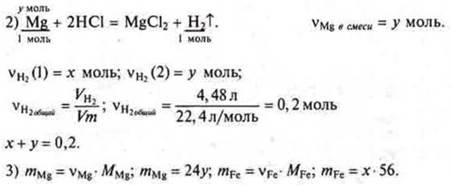
We create the equation 56x + 24U = 8.
4)
a system of equations ![]()
Solving a system of equations:
x = 0.1 mol, mFe= 5.6 g.
Y = 0.1 mol, mMg= 2.4 g.
Answer: mFe = 5.6 g; mIU= 2.4 g.
(Students are asked to solve problem # 38 at home.)
III. Learning new material
Plan of presentation
1. Chemical elements — non-metals:
a) the Position of elements-nonmetals in the Mendeleev PSC.
b) Features of the structure of nonmetal atoms. Possible degrees of oxidation. Change of nemetallicheskie in periods and in groups main group.
C) Electronegativity (EO). The change of EO in the period and in the groups of the main subgroups of the PSE with an increase in the charge of the atom's nucleus.
2. Simple substances-non-metals:
a) a Chemical bond, the crystal lattice.
b) Physical properties of non-metals; allotropy.
C) Chemical properties of non-metals.
d) Receipt and application.
In PSHE nonmetals located to the right and up from the diagonal line drawn from boron to astatine. That is, they are located in the main subgroups of groups III, IV, V, VI, VII, and VIII. On the outer energy level of atoms of the nonmetals have four or more electrons four. Hydrogen and helium are s-elements, and all other nonmetals are p-elements. Inert gases have a completed external energy level, all other nonmetal atoms have an external energy level close to completion. Before stability, they accept the missing number of electrons, thereby acquiring a similarity to the external energy level of inert gases. Octet — 8 electrons, He (helium) has two.
In periods with increasing charge of the nucleus of an atom of an element increases the number of electrons on the outer energy level, resulting in the atomic radius of an atom decreases, increasing nemetallicheskie — the ability to accept electrons. In groups, the main subgroups, the number of energy levels increases with the increase in the charge of the atom's nucleus, i.e. the atomic radius of the atom increases, and the number of electrons at the external energy level remains unchanged. The end groups of the main subgroup of nemetallicheskie decreases.
The ability to accept electrons is a manifestation of the oxidative properties of the atoms of elements. Thus, the oxidative properties of nonmetals are enhanced by the end of the period and by the beginning of the main subgroup group.
The important concept of electronegativity (EO) is used for quantitative characterization. The maximum EO value is F, which is equal to 4. It is the most non-metallic element, the strongest oxidizer.
The remaining nonmetals have an EO value that changes from 2 to 4, and according to these values, they are placed in a row of EOS (flyleaf of the textbook, p.229).
Comparing the values of EO of non-metals, we conclude that the same element, depending on the location in the row of EO in relation to some elements, manifests itself as an oxidizer, to others — as a reducing agent.
Example: S — sulfur, exhibits oxidative properties in relation to elements located to the left; in a series of EO's exhibits reducing properties towards the elements located to the right in the row of EI.
Possible nonmetal Cations depend on the structure of atoms in the main and in the excited state.
Example: P-phosphorus.
Ground state:
P + 15; 1s22s22p63s23p33d0
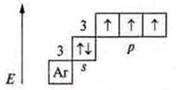
Until the stability of the external energy level, the phosphorus atom takes 3E-.
![]()
CP -3, minimum. P is the oxidizer. Can give away 3E-.
![]()
S. O. +3 intermediate. P-reducing agent and oxidizer.
At the phosphorus atom is in the third energy level, possibly opening the d-sublevel in the excited state may steaming 3s2electrons due to external energy (e.g. light).
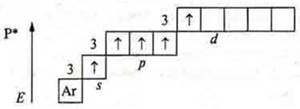
![]()
reductant.
CP +5, the maximum, it corresponds to the group number.
Conclusion:
Depending on the structure of the atom, nonmetals can take the minimum, maximum
and intermediate Forms. Taking the minimum CP, they act as oxidizers: ![]()
![]() When
they accept maximum CP, they act as reducing agents:
When
they accept maximum CP, they act as reducing agents: ![]()
Nonmetal atoms form molecules of simple substances, usually diatomic. Chemical bond-covalent nonpolar crystal lattices can be atomic or molecular.
Example:
H2— hydrogen, O2-oxygen; CL2-chlorine; I2-iodine, C-diamond;
H2, O2, CL2-gases, molecular crystal lattices;
I2-solid;
C-diamond is an atomic crystal lattice.
Nonmetal atoms that have several unpaired electrons can form several molecules of simple substances. This phenomenon is called allotropy, and simple substances are called allotropic modifications.
Example:
O2— oxygen, O3-ozone;
S8-crystalline sulfur, Sn-plastic sulfur;
P — red phosphorus, P4-white phosphorus;
C — carbon, diamond, graphite, carbine, fullerenes.
The more unpaired electrons there are in an atom, the greater the possibility of forming allotropic modifications.
The physical properties of non-metals and metals differ significantly.
For example, the color: I2— purple (pairs), black with a metallic luster (TV.); VG2— brown; P4 — pale yellow, P-red; O2(жидкliquid) — blue; CL2(gas) — green.
Depending on the type of crystal lattice, nonmetals may have both common and distinctive physical properties. Gaseous nonmetals, liquid bromine, crystals-insulators, crystals-are not plastic; most nonmetals have a metallic luster: I2, Si, C (graphite).
Chemical properties of non-metals should be considered as redox, according to their EO. However, in relation to hydrogen and metals, they are all oxidizers.
Example:
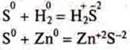
In
both cases![]() ,
S-sulfur is an oxidizer.
,
S-sulfur is an oxidizer.
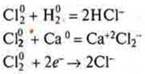
SL20-chlorine, oxidizer.
![]()
N20-nitrogen,
oxidizer: ![]()
If we consider the interaction of non-metals with each other, then we should take into account their EO. Example:
![]()
N2-nitrogen,
reducing agent: ![]()
Non-metals interact with complex substances — inorganic and organic, acting as oxidizers or reducing agents.
Experiment: Nonmetals-oxidizing agents.

b) combustion reaction:
![]() O2is
the oxidizing agent.
O2is
the oxidizing agent.
![]() O2is
the oxidizing agent.
O2is
the oxidizing agent.
![]() CL2(chlorine)
is an oxidizing agent.
CL2(chlorine)
is an oxidizing agent.
Experiment: non-Metallic reducing agents.
![]() H20-reducing
agent,
H20-reducing
agent,
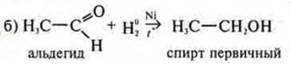 N20is
the reducing agent.
N20is
the reducing agent.
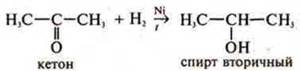 H2is
a reducing agent.
H2is
a reducing agent.
In conclusion, the teacher and students make a table.
Chemical properties of nonmetals
|
Nonmetals |
|
|
Oxidizing agents |
Reducing |
|
agents a) + b metals) + non-metals with less than EO b) + complex substances (oxides, acids, salts, hydrogen compounds, organic substances) |
and) + non-metals with a higher density EO b) + complex substances (salts, oxides, acids, organic compounds) |
Getting non-metals:
a) Electrolysis of molten salts:
halogenides:
![]()
b) the Displacement of salts from the more active halogen:
![]()
C) from liquid air: O2; N2; O3;
d) in nature: C, S;
d) S is the silicon from the SiO2:
![]()
e) P-phosphorus
![]()
g) Se-selenium and Te-tellurium from the waste of sulfuric acid production in the form of impurities in the composition of ore FeS2.
h) H2-methane conversion rate:
hydrogen: ![]()
I)
V-boron: ![]()
III. Homework assignment
§ 19 to p. 236, no. 3, 5, p. 242, task no. 12.
IV. Consolidation
To perform the task No. 10, p. 242.
Perform: I row — "a)", II row — " b)". III row — " b)".
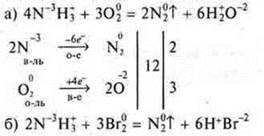
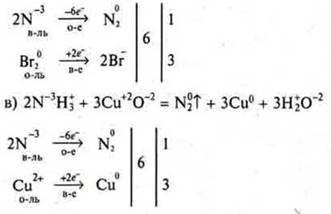
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.