
Problems solving
Elementary Level Questions
1. The
maximum efficiency of a heat engine that operates between temperatures of 1500
K in the firing chamber and 600 K in the exhaust chamber is most nearly
(A) 33% (B) 40% (C) 60% ( D) 67% (E) 100%
2. In
each cycle of a certain Carnot engine, 100 joules of heat is absorbed from the
high‑temperature reservoir and 60 joules is exhausted to the low‑temperature
reservoir. What is the efficiency of the engine?
(A) 40% (B) 60% (C) 67% (D) 150% (E) 167%
3. The theoretical (Carnot) efficiency of a heat engine operating between 600ºC and 100ºC is:
(A) 16.7% (B) 20.0% (C) 42.7% (D) 57.3% (E) 83.3%
4. A heat engine takes in 200 J of thermal energy and performs 50 J of work in each cycle. What is its efficiency?
(A) 50 % (B) 40 % (C) 25 % (D) 20 % (E) 12 %
Intermediate Level Questions
5. An engine operates on the cycle shown in the PV diagram below. The working substance of the engine is an ideal monatomic gas. The processes A → B and C → D are isobaric, while processes B → C and D → A are isochoric.
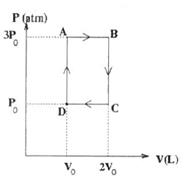
What is the efficiency of a Carnot engine operating between the same maximum and minimum temperatures as this engine?
(A) 1/6 (B) 1/3 (C) 1/2 (D) 3/5 (E) 5/6
6. What is the actual efficiency of this engine?
(A) 1/6 (B) 4/21 (C) 5/17 (D) 7/19 (E) 8/15
7. What would be the efficiency of the heat engine diagramed as shown below?
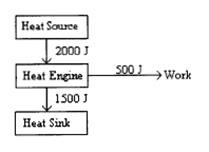
(A) 300 % (B) 133 % (C) 75 % (D) 33 % (E) 25 %
Advanced Level Questions
8. A proposed ocean power plant will utilize the temperature difference between surface seawater and seawater at a depth of 100 meters. Assume the surface temperature is 25° Celsius and the temperature at the 100‑meter depth is 3° Celsius.
a. What is the ideal (Carnot) efficiency of the plant?
b. If the plant generates useful energy at the rate of 100 megawatts while operating with the efficiency found in part (a), at what rate is heat given off to the surroundings?
The diagram below represents the Carnot cycle for a simple reversible (Carnot) engine in which a fixed amount of gas, originally at pressure po and volume Vo follows the path ABCDA.
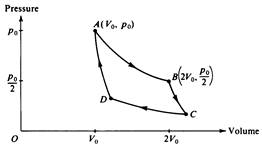
c. In the chart below, for each part of the cycle indicate with +, ‑, or 0 whether the heat transferred Q and temperature change DT are positive, negative, or zero, respectively. (Q is positive when heat is added to the gas, and DT is positive when the temperature of the gas increases.)
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.