
|
Long-term plan section: Fundamentals of thermodynamics |
School: |
|||||||||||||||||||||||||||||||||||||
|
Date: _______________________ |
Teacher’s Name: |
|||||||||||||||||||||||||||||||||||||
|
Класс: 10 ____ |
Number of attendees: ______ |
Absentees: ____ |
||||||||||||||||||||||||||||||||||||
|
Lesson Topic |
Reversible and irreversible processes. Entropy. The second law of thermodynamics. |
|||||||||||||||||||||||||||||||||||||
|
Learning objectives that are achieved in this lesson (link to the curriculum) |
To describe the Carnot cycle for an ideal heat engine; To apply the formula for the efficiency of a heat engine for solving problems. |
|||||||||||||||||||||||||||||||||||||
|
Lesson objectives |
- knows the wording of the second law of thermodynamics; - can explain the meaning of the second law of thermodynamics; - applies the second law of thermodynamics in the analysis of thermodynamic processes. |
|||||||||||||||||||||||||||||||||||||
|
Evaluation Criteria |
- knows the wording of the second law of thermodynamics; - can explain the meaning of the second law of thermodynamics; - applies the second law of thermodynamics in the analysis of thermodynamic processes. |
|||||||||||||||||||||||||||||||||||||
|
Language objectives |
Vocabulary and terminology specific to the subject:
|
|||||||||||||||||||||||||||||||||||||
|
Instilling values |
Humanity Throughout the lesson, instill openness, cooperation, respect, tolerance in relationships with classmates The imparting of values is carried out through / through a discussion of the key points of the topic in groups. |
|||||||||||||||||||||||||||||||||||||
|
Interdisciplinary communication |
Relationship with mathematics: working with formulas, the standard form of numbers, physical quantities and their units of measurement. |
|||||||||||||||||||||||||||||||||||||
|
Key skills |
Critical thinking through the creation of a problem situation |
|||||||||||||||||||||||||||||||||||||
|
Preliminary knowledge |
Internal energy, ways of changing internal energy, The first law of thermodynamics. |
|||||||||||||||||||||||||||||||||||||
|
Lesson Proper |
||||||||||||||||||||||||||||||||||||||
|
Scheduled Lesson Stages |
Scheduled Lesson Activities |
Resources |
||||||||||||||||||||||||||||||||||||
|
Beginning 10 min. |
(I) Determine the amount of substance of the gas (60,2 mol) (II) Determine the gas temperature and volume in the second state. (1600 К, 10 м3) (III) Determine the volume of gas in state 3. (10 м3) (IV) What work did the gas do during the isobaric expansion? (4∙105 J)
Question: How has the internal energy of a gas changed for a cycle? Can we say that then, during the cycle, the gas received an amount of heat equal to 4 * 105 J? (no, since in the area 3-1 external forces performed work on the gas, and during the isochoric cooling it gave off heat). It is very important to speak through each stage of work on a structural task !!!!
Discussion of the lesson topic: Reversible and irreversible processes. Entropy. The second law of thermodynamics.
Learning objectives: o knows the wording of the second law of thermodynamics; o can explain the meaning of the second law of thermodynamics; o applies the second law of thermodynamics in the analysis of thermodynamic processes. |
Worksheet.
Slides 1-2
Slides 3-5
|
||||||||||||||||||||||||||||||||||||
|
Middle 27 min. |
At the beginning of the lesson, return to the concept of reversible and irreversible cycles. (Т) What cycles do we most often encounter in real life? What cycles do heat engines work for? In the absence of friction, all mechanical processes would proceed reversibly. Thus, the equilibrium reversible processes are an abstraction due to existing friction and heat transfer. If this were possible, the process would be reversible. And what is a reversible process? Reversible is a process that allows the system to return to its original state without any changes in the environment.
Ask the class:Did it happen to you that you broke an egg or accidentally broke a cup or plate? But have you ever seen how a broken egg or a broken plate becomes whole again? Why not? Why are some phenomena possible and others not? (Т) The answer is entropy.
Entropy determines system disorder — the number of different ways particles can be located in a system.A broken egg has more entropy than a whole egg.Solids have low entropy, liquids have higher entropy than solids, and gases have the highest entropy.
Entropy increases with temperature as well as with volume.
In thermodynamics, entropy is a quantity determined by the ratio:
where S is the entropy. Those the entropy change is equal to the amount of heat transferred in the process to the temperature at which this process took place.
(G) Task 2. Solve the task in pairs. When deciding students Discuss the solution in pairs, then hold a general discussion with the teacher. Suppose a cup of coffee with a temperature 80 0С cooling in a room with a temperature 20 0С, loses Q =1000 J heat. А) Determine the entropy change ΔS cup of coffee.
В) Determine the entropy change ΔS air in the room.
С) Determine the total entropy of the system.
We bring students to the independent formulation of a conclusion.
(Т) Make a conclusion: The total entropy of the system must always increase. This means that events like breaking eggs are irreversible.
An egg cannot spontaneously return to a more ordered state.
A hot cup of coffee is cooled .... ..... but a cold cup of coffee will never warm up spontaneously.
If the cooling were reversible, cold coffee could spontaneously heat up, receiving thermal energy from the air.
The processes associated with the flow of heat are irreversible.
Heat is transferred spontaneously from bodies with a higher temperature to bodies with a lower temperature, but never vice versa. It is not difficult to break eggs and make scrambled eggs, but it is impossible to recreate raw eggs from ready-made eggs. The smell of an open bottle of perfume fills the room - but you cannot collect it back into the bottle. Why?
The reason for such irreversibility of the processes occurring in the Universe lies in the second law of thermodynamics, which, for all its seeming simplicity, is one of the most difficult and often misunderstood laws of classical physics.
The second law of thermodynamics says that the total entropy of a closed system can only increase or remain unchanged (Boltzmann Equation).
The second law of thermodynamics fixes the direction of time only forward, in the direction of increasing entropy.
The second law is sometimes called the "arrow of time."
(W) Ask the class: Analyze and explain everyday examples illustrating the second law of thermodynamics: entropy increase.
Examples of answers: A broken window will never spontaneously recover. Spilled milk will never flow back into the glass. A new deck of cards can never be shuffled back in the original order.
(T) Experience shows that different types of energy are not equivalent in terms of the ability to transform into other forms of energy. The second law of thermodynamics has several formulations.
Clausius’ Formulation: Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time.
Thomson’s formulation: it is impossible to carry out such a periodic process, the result of which would be the performance of work at the expense of heat, taken from one of a certain body. If, for example, a steam engine performs work due to the heat received from the steam boiler, then the work done is not the only result of the process, since part of the heat necessarily goes into the atmosphere along with the exhaust steam.
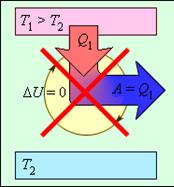 This formulation imposes a restriction on the
transformation of internal energy into mechanical energy. It is impossible to
build a car (a perpetual motion machine of the second kind) that would do
work only by obtaining heat from the environment. This formulation imposes a restriction on the
transformation of internal energy into mechanical energy. It is impossible to
build a car (a perpetual motion machine of the second kind) that would do
work only by obtaining heat from the environment.
(G) Task 3. Solve tasks in pairs. After joint discussion and solving the assignment, the students compare their answers with the answers of the next couple in pairs.
1. The irreversible process is a) a process that can occur both in the forward and in the opposite direction b) a process that does not reverse spontaneously c) a process that takes place without the influence of external bodies
2. Choose a formulation that is not relevant to the second law of thermodynamics. a) The amount of heat supplied to the system goes to change its internal energy and to perform the system work. b) Heat itself cannot move from a colder body to a warmer one. c) In nature, a process is impossible, the only result of which would be mechanical work done by cooling the heat reservoir d) It is impossible to design a machine that will only transfer heat from a colder body to a warmer one.
3. The second law of thermodynamics reflects ... a) orientation of thermodynamic processes in closed systems b) energy conservation in thermodynamic processes c) the transformation of energy from one type to another in thermodynamics d) the ability of internal energy to spontaneously transform into work
(I) Task 4. Individual problem solving. 1. Determine the entropy change when melting 63.5 g of copper, if the heat of melting of copper is equal to 12980 J/mol, and the melting point of copper 1083 °С.
(G) Task 5. Solve tasks in pairs. After a joint discussion and solving the assignment, the students compare their answers with the answers of the next couple, prove the correctness and falsehood of the statements. Determine which statements are true and which are false.
Internal gas energy increased by 550 J.
|
Slide 6
Slides 7-12
Slides 13-14
Slides 15-19
Worksheet.
Worksheet.
Worksheet.
Worksheet.
|
||||||||||||||||||||||||||||||||||||
|
End 3 min |
Reflection on the assessment of the level of learning students. "What is your level?" |
|
||||||||||||||||||||||||||||||||||||
|
Differentiation - how do you plan to provide more support? What tasks do you plan to set for more capable students? |
Grading - How do you plan to check students' level of learning? |
Health &
Safety |
||||||||||||||||||||||||||||||||||||
|
I plan to support them through a general discussion of the results of working with text with Internet sources, suggested text questions, and individual counseling. |
Pairing, individual counseling; self-assessment |
Creating a favorable psychological atmosphere, the compliance of tasks to the level of preparedness of students, the change of activities, counseling. |
||||||||||||||||||||||||||||||||||||
|
What tasks do you plan to set for more capable students? |
Providing advice to students who have difficulty |
|||||||||||||||||||||||||||||||||||||
|
Students will: Most students will:
Some learners will be: |
- explain the meaning of the first and second laws of thermodynamics.
Find the entropy of a system as a result of a chemical process based on a chemical formula. |
|||||||||||||||||||||||||||||||||||||
|
Lesson reflection |
Use this section to reflect on the lesson. Answer the most important questions about your lesson from the left column. |
|||||||||||||||||||||||||||||||||||||
|
Were the objectives of the lesson / learning objectives realistic? |
|
|||||||||||||||||||||||||||||||||||||
|
Have all students reached the DH? If not, why not? Is the differentiation correct in the lesson? Have the lesson temporal stages been sustained? What were the deviations from the lesson plan and why? |
|
|||||||||||||||||||||||||||||||||||||
|
Overall rating
|
||||||||||||||||||||||||||||||||||||||
|
What two aspects of the lesson went well (think about both teaching and learning)? 1:
|
||||||||||||||||||||||||||||||||||||||
|
2:
|
||||||||||||||||||||||||||||||||||||||
|
What could contribute to improving the lesson (think about both teaching and learning)? 1:
2:
|
||||||||||||||||||||||||||||||||||||||
|
What did I find out during the lesson about the class or the achievements / difficulties of individual students, what should I look for in subsequent lessons?
|
||||||||||||||||||||||||||||||||||||||
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.