
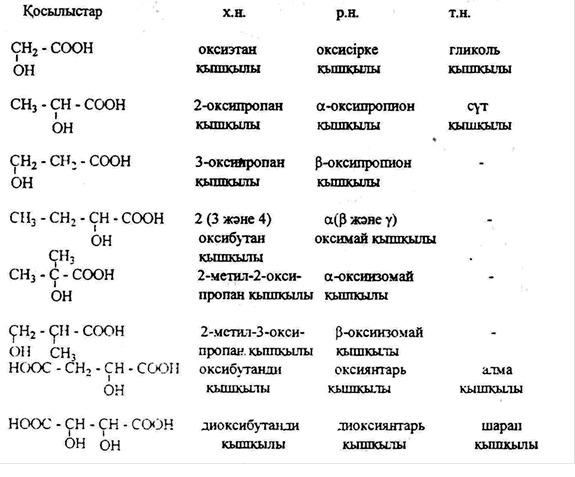
Oxycarboxylic acids
Plan:
1. homologous series of oxyacids. Isomerism. Nomenclature.
2. physical and chemical properties of oxic Acids
3. Production and application of Oxynoacids.
In the molecule, in addition to carboxylic subgroups, oxyacids are called, derivatives of carboxylic acids containing one or more hydroxyl subgroups.
Depending on the number of hydroxyl groups and carboxyl oxycise as follows: oxycarbon (one hydroxyl, one carboxyl), oxidisable (one hydroxyl, two carboxyl), aksionovo (one hydroxyl group, three carboxyl), dioxolane (two hydroxyl, one carboxyl), dioxygenase (two hydroxyl, two carboxyl).
Isomerism and nomenclature
Isometry consists of three types of isometry.
1. chain isometry depending on the branching of the carbon chain in the molecule.
2. state isomerism determined by the position of the hydroxyl (oxy) subgroup in the carbon chain. As a rule, the position of the hydroxyl topshi comes from the Greek letters (α, β, γ).)
Physical and chemical properties of oxides
Oxy-oxidations are colorless water-soluble liquids or crystalline substances that have a sour taste.
Oxy acids are characterized by the usual properties of carboxylic acids, i.e. the formation of salts, esters, halides, and amides.
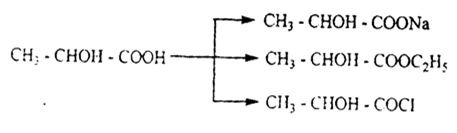
The hydroxyl subgroup of oxy-acids is characterized by the usual properties of alcohols, i.e. the formation of ALCOHOLATES, simple and complex esters, and the exchange of hydroxyl with halogen:
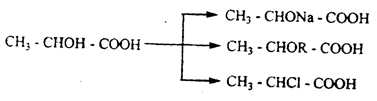
In all reactions, the interaction of carboxyl and hydroxyl subgroups is observed. In accordance with this, oxy-acids have their own properties.
1. in a solution of mineral liquefied acids, α-oxides break down into aldehydes or ketones and formic acid:
![]()
2. due to the action of oxidizing agents, such as hydrogen iodide, α-hydroxy acids are reduced to carboxylic acids:

3. reactions of water release from Oxynoacids occur in different directions according to the purpose of acids:
a) when heated, α-oxy
acids release water and give cyclic esters called lactides: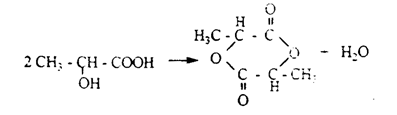
ә) β-hydroxy acids secrete water and form unsaturated acids:
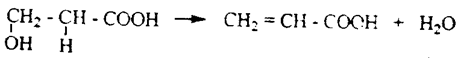
b) when heated, γ and δ-hydroxy acids form cyclic esters, i.e. lactones:
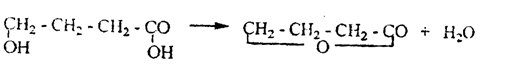
Oxy-acids (except glycolic acids) have a special interesting property of turning the plane of polarization of the light beam.
If light radiation penetrates through the Nicole prism, which consists of two Iceland spar crystals, then all vibrations, except those in a certain plane, are absorbed in the prism. Light passing through a prism is called flat polarized radiation.
If a polarized beam penetrates a solution of one substance, the solution of the substance rotates the plane of radiation polarization by a certain angle (Fig. 16). Optically active substances are substances that can reverse or change the plane of radiation polarization by a certain angle. Optically active substances can turn the radiation plane to the right or left. Indicate the direction of rotation ( + ) and ( - ) signs, respectively.
Optical activity is a common phenomenon in organic compounds. Optically active compounds include oxy-acids, carbohydrates, and proteins.
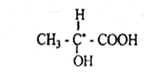
Here, the Central carbon atom is associated with hydrogen, carboxyl, hydroxyl, and methyl furnaces, i.e., with four different substituents. Therefore, the Central carbon atom in lactic acid is asymmetric (in the Formula it is denoted by an asterisk). Therefore, lactic acid shows optical activity. In fact, there are three different forms of lactic acid in nature that differ from polarized radiation.
There are two ways to place four different substituents from the lactic acid Formula in space. Figure 17 shows tetrahedral and ball-rod models of lactic acid molecules from two different substituent arrangements in space.
Two milk-containing lactic acids, their spatial structure is different. They can be based on the spatial structure of the image of each other in the mirror, and the access of the right hand to the left hand.
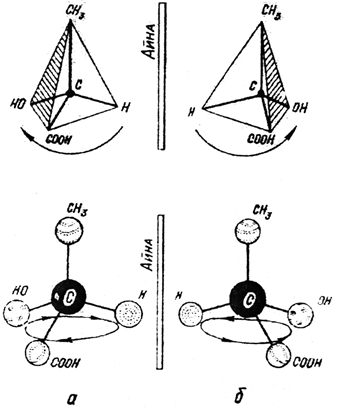
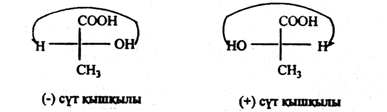
Both molecules have the same composition, physical and chemical properties, but they differ in optical properties. One of the molecules rotates the polarized beam to the right, and the other rotates the same angle (as many degrees) to the left. Only compounds that have a difference in the sign of rotation are said to be optical isomers.
Thus, the plane of polarization of the beam clockwise has lactic acid and (+) lactic acid and (-) lactic acid.
Ways to get actinocyclus
Since the oxide molecule has hydroxide and carboxylic subgroups, they are obtained by introducing a carboxylic subgroup into alcohols, and by introducing a hydroxyl subgroup into acids.
1. when heating α-Halocarboxylic acids with water, it is hydrolyzed and gives oxyacids:
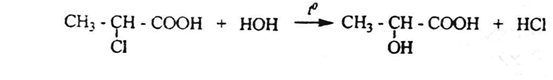
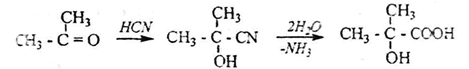
2. oxynitriles synthesized from aldehydes or ketones are also obtained by hydrolysis of α-oxy acids:
3. β-hydroxy acids are easily obtained by the reformed method. In this reaction, the esters of α-halocarbonic acids react with aldehydes or ketones in the presence of marsh. β-hydroxycyclic ether forms zinc alcoholism, which then breaks down under the influence of water and gives β-hydroxyacid:
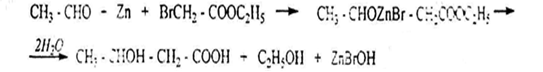
4. by hydration of unsaturated acids, β, γ, and δ – hydroxy acids are obtained instead of the double bond. The reaction occurs in the presence of acid catalysts:
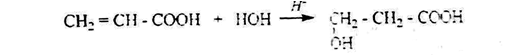
5. oximosilane you can get acetaldehyde or oxidation of glycols:
![]()
6. doxiciclina and dicarboxylic acids oxidize unsaturated carboxylic acid in the case of the reaction of Wagner:
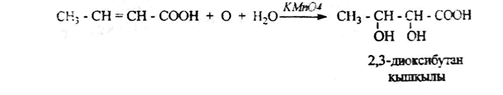
The most important representatives of axinom
Glycolic acid-a colorless crystalline substance, well soluble in water.
In nature, it is found in the composition of non-specific fruits, such as grapes. Synthetic glycolic acid is obtained from Chloroacetic acid.
Glycolic acid is used in organic synthesis.
Lactic acid is a colorless, very hygroscopic solid, but it usually forms a viscous liquid.
The first lactic acid was discovered in 1780 by the German scientist Scheele in the composition of fermented milk, according to which it was called lactic acid. Later it turned out that it is formed due to the opening of food products. it contains the composition of yeast cabbage, pickled cucumber, cheese, etc. Lactic acid has three kinds: the two optical antipodes (enantiomer) and the racemate. Their exact configuration is determined: lactic acid with a turn to the left belongs to the row (R-configuration), to the right – to the L-row (S-configuration).
In the dairy industry.
![]()
Mammals are used in the food, leather, and textile industries.
Malic acid is a colorless crystalline substance that is highly soluble in water and has a pleasant sour taste. It has two optical antipodes of malic acid and an optical inactive racemate:

Lisomer S-refers to the configuration, D-isomer R-configuration, according to which you can write as S ( – ) – malic acid and R (+) - malic acid.
Malic acid is used in organic synthesis and medicine.
Tartaric acids are a colorless crystalline substance that is highly soluble in water and has a pleasant sour taste. Their molecules contain two asymmetric carbon atoms. Each asymmetric atom corresponds to two antipodes and one racemate. Therefore, tartaric acids must have four optical isomers and two racemates. But to tartaric acid correspond optical inactive racemates (grape acid) and mesosarapic acids optical inactive, consisting of two optical ( + ) and ( - ) isomers, equal admixtures of both:
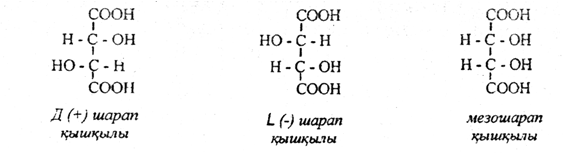
The d-isomer refers to the r, R-configuration, L-isomer S, S – configuration,
and mesosarapic acid R, S-configuration.
Tartaric acid and its salts (tartrates) are widely used. Tartaric acid is used in the food industry and paint.
Citric acid is a colorless crystalline substance that is crystallized from water as a monohydrate:
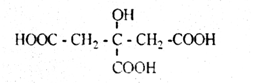
Citric acid occurs naturally with lemon juice, tobacco leaves, etc. In production, it is obtained by fermentation of glucose (citric acid).
Citric acid is used in the food industry and pharmacology. Citric acid esters are used as plasticizers.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.