
Exercises
1. In a 5 m3 boiler pumped water weighing 20 kg. The contents of the boiler was heated to a temperature of 453 K. Find the mass and pressure of steam in the boiler.
2. Two kilograms of water at 25°C are placed in a piston cylinder device under 3.2 MPa pressure as shown in the diagram (State (1)). Heat is added to the water at constant pressure until the temperature of the fluid reaches 350°C (State (2)). Determine the final volume of the fluid at state (2).
3. A piston-cylinder device contains a saturated mixture of steam and water having a total mass of 0.5 kg at a pressure of 160 kPa and an initial volume of 100 liters. Heat is then added and the fluid expands at constant pressure until it reaches a saturated vapor state.
· a) Draw a diagram representing the process showing the initial and final states of the system.
· b) Sketch this process on a P-v diagram with respect to the saturation lines, critical point, and relevant constant temperature lines, clearly indicating the initial and final states.
· c) Determine the initial quality and temperature of the fluid mixture prior to heating.
· d) Determine the final volume of the steam after heating.
Note: 1000 liters - 1 m3.
A pressure cooker allows much faster (and more tender) cooking by maintaining a higher boiling temperature of the water inside. It is well sealed, and steam can only escape through an opening on the lid, on which sits a metal petcock. When the pressure overcomes the weight of the petcock, the steam escapes, maintaining a constant high pressure while the water boils.
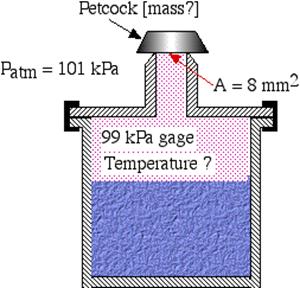
Assuming that the opening under the petcock has an area of 8 mm2, determine
Note: Assume that the atmospheric pressure is 101 kPa. Draw a free body diagram of the petcock.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.