
Theoretical material for the lesson, definitions for concepts
The magnetic moment of a system measures the strength and the direction of its magnetism. The term itself usually refers to the magnetic dipole moment. Anything that is magnetic, like a bar magnet or a loop of electric current, has a magnetic moment. A magnetic moment is a vector quantity, with a magnitude and a direction. An electron has an electron magnetic dipole moment, generated by the electron's intrinsic spin property, making it an electric charge in motion. There are many different magnetic behavior including paramagnetism, diamagnetism, and ferromagnetism.
An interesting characteristic of transition metals is their ability to form magnets. Metal complexes that have unpaired electrons are magnetic. Since the last electrons reside in the d orbitals, this magnetism must be due to having unpaired d electrons. The spin of a single electron is denoted by the quantum number msms as +(1/2) or –(1/2). This spin is negated when the electron is paired with another, but creates a weak magnetic field when the electron is unpaired. More unpaired electrons increase the paramagnetic effects. The electron configuration of a transition metal (d-block) changes in a coordination compound; this is due to the repulsive forces between electrons in the ligands and electrons in the compound. Depending on the strength of the ligand, the compound may be paramagnetic or diamagnetic.
Ferromagnetism (Permanent Magnet)
Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets. This means the compound shows permanent magnetic properties rather than exhibiting them only in the presence of an external magnetic field (Figure 11). In a ferromagnetic element, electrons of atoms are grouped into domains in which each domain has the same charge. In the presence of a magnetic field, these domains line up so that charges are parallel throughout the entire compound. Whether a compound can be ferromagnetic or not depends on its number of unpaired electrons and on its atomic size.
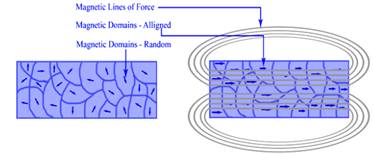
Figure 11: Ferromagnetism (a) nonmagnatized material and (2) Magnetized material with corresponding magnetic fields shown.
Ferromagnetism, the permanent magnetism associated with nickel, cobalt, and iron, is a common occurrence in everyday life. Examples of the knowledge and application of ferromagnetism include Aristotle's discussion in 625 BC, the use of the compass in 1187, and the modern-day refrigerator. Einstein demonstrated that electricity and magnetism are inextricably linked in his theory of special relativity.
Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. The unpaired electrons are attracted by a magnetic field due to the electrons' magnetic dipole moments. Hund's Rule states that electrons must occupy every orbital singly before any orbital is doubly occupied. This may leave the atom with many unpaired electrons. Because unpaired electrons can spin in either direction, they display magnetic moments in any direction. This capability allows paramagnetic atoms to be attracted to magnetic fields. Diatomic oxygen, O2O2 is a good example of paramagnetism (described via molecular orbital theory). The following video shows liquid oxygen attracted into a magnetic field created by a strong magnet:
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Magnetic_Properties
As shown in the video, molecular oxygen (O2O2 is paramagnetic and is attracted to is paramagnetic and is attracted to the magnet. In contrast, molecular nitrogen, N2N2, has no unpaired electrons and is diamagnetic; it is therefore unaffected by the magnet. Diamagnetic substances are characterized by paired electrons, e.g., no unpaired electrons. According to the Pauli Exclusion Principle which states that no two electrons may occupy the same quantum state at the same time, the electron spins are oriented in opposite directions. This causes the magnetic fields of the electrons to cancel out; thus there is no net magnetic moment, and the atom cannot be attracted into a magnetic field. In fact, diamagnetic substances are weakly repelled by a magnetic fieldas demonstrated with the pyrolytic carbon sheet in Figure 22.

Figure 22: Levitating pyrolytic carbon: A small (~6 mm) piece of pyrolytic graphite levitating over a permanent neodymium magnet array (5 mm cubes on a piece of steel). Note that the poles of the magnets are aligned vertically and alternate (two with north facing up, and two with south facing up, diagonally). Image used with permission from Wikipedia.
Additional guidelines for organizing a lesson
Lesson starts with introducesing the topic of day and spells out the learning outcomes they will possess after the study. Acquaint students with the following issues:
• The theme of the lesson
• The objectives of the lesson
• The criteria of success for the lesson
• The plan of events for the lesson
• Pre-teach the subject specific vocabulary.
Learners will share their experiences with magnetic properties of materials. Classify materials as magnetic, non-magnetic, ferromagnetic, paramagnetic, diamagnetic from assorted list provide by the teacher.
Then students can deduce topic of the lessen and objectives, for clarification you can show topic and the learning objectives on the presentation.
Then Subject-specific vocabulary & terminology will be presented to the students and their activities during the research work will be explained.
Then teacher will explain the properties and applications of magnets. Describe, discuse, and explain the applications of magnets. Ask learners to develop presentations on neodymium magnets, sensors, seismographs, and metal detectors. Give questions on a worksheet.
Learners will discuss their experiences with applications of magnetic substances. Watch videos on left-hand rule, Ampere force, and Lorentz force. Answers questions about on the videos. Work in groups to develop presentations about neodymium magnets, sensors, seismographs, and metal detectors. Attempt questions on the worksheet.
Properties of magnets:
https://www.youtube.com/watch?v=-hGdR-kx8FM
Magnets in daily life:
https://www.youtube.com/watch?v=4pz37NMAH3Y
Then teacher:
• Highlights key concepts, definitions, and equations learnt using the concept map.
• Asks students to do questions on the worksheet provided.
• Looks forward to the next lesson.
Students would:
• Attempt the questions given by the teacher.
• Summarize the main concepts, definitions, and equations learnt.
• Reflect on their own learning.
• Evaluate their own work and the work of their classmates.
Extension Work:
• Complete the flipped reading and research assignment before the next lesson.
Homework:
• Complete the specified thinking tasks for this lesson.
• Should complete worksheet given by teacher
Additional multilevel (on differentiation) tasks
The teacher assigns questions 1-4 to weak students, questions 5-6 to the average students, question 1 to the strong students.
Recommendations for formative assessment
Individually work, students will check themselves teacher prepared answers.
Answers, criteria for assignments, additional materials for the lesson
Teacher will prepare answer using theory material
1. A
2. B
3. A
4. C
5. D
6. C
7. A
8. B
9. B
10. B
11. C
12. B
13. D
14. B
15. B
16. A
17. B
18. C
19. B
20. D
Скачано с www.znanio.ru
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.