
Questions on brainstorming
- What do you understand as macroscopic bodies?
- What parameters are called macroscopic?
- What is the physical meaning of temperature?
- What kind of temperature scales do you know?
- What is the basic equation of the molecular-kinetic theory?
- What is the physical meaning of the Boltzmann constant?
- How is the average kinetic energy of chaotic motion of molecules related to the temperature of the system?
Problems about PV = nRT
1. What is the volume of 1 kg of nitrogen gas (diatomic) at 240c and 0.98 atm? 888L
2. What is the mass of 16.5L of helium gas at 210C and 1.03 atm? 2.81 g
3. What is the density of hydrogen gas at -100C and 0.95 atm? 88mg/L
4. What is the mass of 6L of oxygen gas at -100C and 0.95 atm? 8.45 g
5. What is the density of neon gas at -50C and 0.97 atm? 0.885 g/L
6. What is the volume of 1 kg of gaseous water at 1250C and 1.02 atm? 1780 L
7. A balloon is filled with pure nitrogen gas. The balloon is determined to have a volume of 0.75 L on a day when the temperature is 200C, and air pressure is 0.85 atm. How many nitrogen molecules are present? 1.57*1022
8. Which of the following graphs shows an incorrect relationship for an ideal gas?
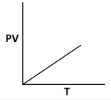
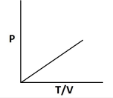
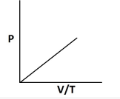
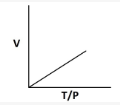
9. By what factor does the volume of an ideals gas change if its temperature increases by 50% and pressure quaruples? 0.375
10. A mixture of gas has a volume of 40 L? a pressure of 2.3 atm? And is at a temperature of 400C. If the gas mixture is 80% nitrogen and 20% oxygen? How many moles of nitrogen are there? 2.9 mol
11. In a 15 L rigid, sealed container? There is 1 mole of an ideal gas/ The container is initially at 250C. If the gas is heating pressure in the container? 1.768 atm
12. A storage tank at STP contains 18.5 kg of nitrogen (a) What is the volume of the tank? (b) What is the pressure if an additional 15.0 kg of nitrogen is added without changing the temperature? a)14.8 m3 / b) 1.82 atm.
13. If 18.75 mole of helium gas is at 10.0°C and a gauge pressure of 0.350 atm, (a) calculate the volume of the helium gas under these conditions. (b) Calculate the temperature if the gas is compressed to precisely half the volume at a gauge pressure of 1.00 atm. A) 0.323 m3/ B) -630C
14. What is the pressure inside a 35.0-L container holding 105.0 kg of argon gas at 385 K? 2.40x10=Pa
15. A
tank contains 26.0 kg of ![]() gas
at a gauge pressure of 8.70 atm. If the oxygen is replaced by helium, how many
kilograms of the latter will be needed to produce a gauge pressure of 7.00 atm?
2.68 kg He
gas
at a gauge pressure of 8.70 atm. If the oxygen is replaced by helium, how many
kilograms of the latter will be needed to produce a gauge pressure of 7.00 atm?
2.68 kg He
Hometask
1) Sketch a PV diagram of the following process: 2.0 m3 of ideal gas at atmospheric pressure are cooled at constant pressure to a volume of 1.0m3 and then expanded isothermally back to 2.0 m3, whereupon the pressure is increased at constant volume until the original pressure is reached.
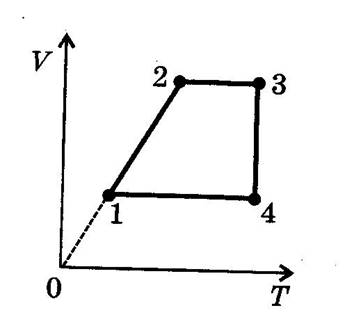
2) Using the V-T diagram below describe what is happening between each of the points:-
· 1-2
· 2-3
· 3-4
· 4-1
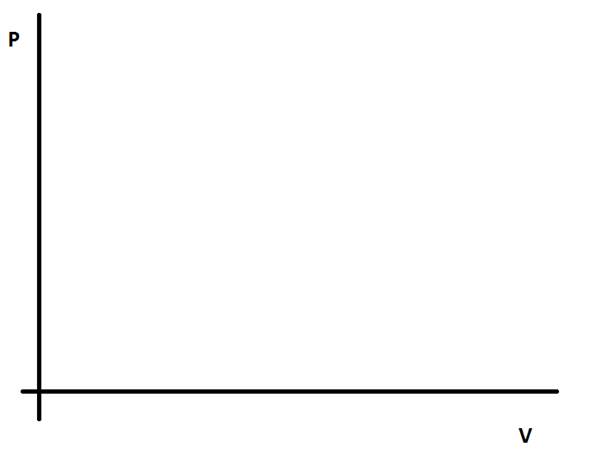
3) Draw onto the P-V graph below showing the changes described in each of the regions 1-4 from the diagram above:-
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.