
 The Carnot Cycle (Mark Scheme)
The Carnot Cycle (Mark Scheme)
Fill in the table with the correct information.
Choose the correct words from the list to fill in the blanks.

|
Parts |
Isoprocesses |
Q |
∆T |
Description |
|
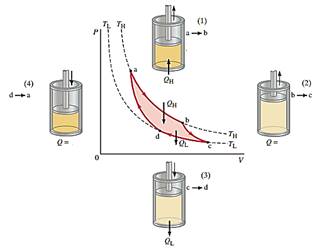
a - b |
Isothermal expansion |
W |
0 |
The gas is first expanded isothermally, with the addition of heat QH, along the path “ab” at temperature TL. |
|
b - c |
Adiabatic expansion |
0 |
- |
Next, the gas expands adiabatically from “b” to “c” – no heat is exchanged, but the temperature drops to TL. |
|
c - d |
Isothermal compression |
W |
0 |
The gas is compressed at constant temperature TL, path “cd”, and heat QL flows out. |
|
d - a |
Adiabatic compression |
0 |
+ |
Finally, the gas is compressed adiabatically, path “da”, back to its original state. |
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.