
Worksheet 2
1. This question is about thermodynamics.
(a) The first law of thermodynamics can be written as the following equation.
Q = ∆U + W
Identify the symbols in this equation.
Q
...............................................................................................................................
...............................................................................................................................
∆U
...............................................................................................................................
...............................................................................................................................
W
...............................................................................................................................
...............................................................................................................................
(3)
(b) A fixed mass of an ideal gas is contained in a cylinder by a piston. The friction between the piston and cylinder wall is negligible.
Two procedures are carried out on the gas. The thermal energy input to the gas is the same in both procedures.
Procedure 1 The gas is heated and expands at constant pressure with the piston free to move. The temperature of the gas increases by 21 K.
Procedure 2 The gas is now brought back to its initial state and again heated with the piston fixed in position. The temperature of the gas increases by 35 K.
(i) State the name of the process in procedure 2.
...............................................................................................................................
...............................................................................................................................
(1)
(ii) Explain why the temperature change is greater in procedure 2 than in procedure 1.
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
(4)
(iii) In procedure 1, ∆U changes by 120 J. Use the first law of thermodynamics to calculate the missing values in the table below.
|
|
∆U / J |
W / J |
Q / J |
|
Procedure 1 |
+120 |
|
+200 |
|
Procedure 2 |
|
|
+200 |
(3)
(Total 11 marks)
2. This question is about gases and thermodynamic processes.
(a) State one way in which a real gas differs from an ideal gas.
...............................................................................................................................
...............................................................................................................................
(1)
(b) The diagram shows how the pressure p varies with volume V of an ideal gas that undergoes a cyclic change of state.
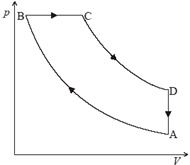
AB and CD are adiabatic changes of state. The pressure at point B is 1.8 × 105 Pa and the change in volume of the gas between B and C is 4.8 ×10–4 m3.
(i) State what is meant by an adiabatic change of state.
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
(1)
(ii) The change in volume of the gas between B and C takes 0.020 s. Determine the power developed during this change of state.
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
(3)
(Total 4 marks)
3. Use full sentences to describe the following two processes. In your answer, mention the shape of the pressure vs volume graphs for each process.
(a) Isobaric process
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
(2)
(b) Isovolumetric process
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
...............................................................................................................................
(2)
(Total 4 marks)
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.