
Review
(D) Demonstration. Electrolysis
a. Pure water b. Ionic Solution
Conclusion:
Pure water does not conducts an electric current.
Ionic solutions conducts a current.
(I) Individual work. Electrolysis
Learners individually investigate about Electrolysis by using internet resources.
(G) Group work. Law of Electrolysis
Divide students into two groups and ask to investigate about Law of Electrolysis:
Ø GROUP 1: Faraday’s First Law of Electrolysis
Ø GROUP 2: Faraday’s Second Law of Electrolysis
After finishing they will present their posters in class and answer the questions.
(T) Teacher explanation. Law of Electrolysis
Teacher expand the topic by explanation.
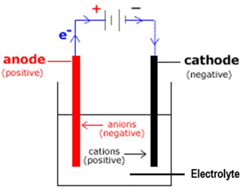
Passing an electric current through a liquid (solutions and fused electrolytes) is called ELECTROLYSIS.
The electric current enters the liquid at the positive plate (called the anode) and leaves it at the negative plate (called the cathode).
In liquids the current is carried by ions. Ions are charged particles (atoms or groups of atoms). Ions can either be positive or negative, the positive ions being attracted to the negative plate and the negative ions to the positive plate.
(W) Whole class work. Filling the gap
1. Electrolysis is the passing of a direct ………………………….. through an ………………. substance that is either molten or dissolved in a suitable solvent, producing chemical reactions at the ……………………….and a decomposition of the materials.
2. Faraday's laws of electrolysis relate the amount of liberated mass at an electrode to the quantity of electricity passing through the electrode. ... Faraday's first law states that the amount of …………….. passed through an …………… is directly proportional to the amount of material liberated from it.
3. Faraday's second law of electrolysis states that, when the same quantity of ……………. is passed through several ……………………., the …...... of the substances deposited are proportional to their respective chemical equivalent or equivalent.
Answer
1. Electrolysis is the passing of a direct electric current through an ionic substance that is either molten or dissolved in a suitable solvent, producing chemical reactions at the electrodes and a decomposition of the materials.
2. Faraday's laws of electrolysis relate the amount of liberated mass at an electrode to the quantity of electricity passing through the electrode. ... Faraday's first law states that the amount of current passed through an electrode is directly proportional to the amount of material liberated from it.
3. Faraday's second law of electrolysis states that, when the same quantity of electricity is passed through several electrolytes, the mass of the substances deposited are proportional to their respective chemical equivalent or equivalent
(f) Formative assessment. Worksheet
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.