
Electronic configurations of atoms of chemical elements and graphic representation of electronic configurations of atoms
Lesson objectives: to teach you how to make electronic formulas of chemical element atoms and their graphical configurations based on the rules and principles of supplementing the electronic shells of atoms: to consolidate your knowledge of the electronic classification of elements: s-, p-, d-, f-family.
Basic concepts; electronic formula, graphic configuration of an electronic formula, paired and unpaired electrons, Pauli principle, Hund's rule (Gund), the Klechkovsky rule Клечковского, the distribution formula, the filling formula; the symmetry of the atom.
Equipment: Mendeleev PSCE; tables of the electronic structure of atoms of chemical elements.
Lesson progress
I. Organizational moment
Setting goals and tasks for the lesson.
II. Checking that homework has been completed correctly
Students had to give a description of the elements № 3, 16, 5, 14, 2.
Students explain the characteristics of elements from the point of view (speaking).
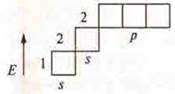
1) Element # 3. Lithium, the charge of the nucleus is +3, the electrons in the atom are 3, n = 2, there are two energy levels in the atom: n = 1, the first energy level;
l = 0; one sublevel; s-sublevel;
ml = 0, one orbital, is spherical.
1s; 1![]()
n = 2, second energy level l = 0.1; l = 0, s-sublevel; ml
= 0, one orbital 1s; 1![]()
l = 1; p-sublevel, ml = +1, 0, -1; three orbitals.
![]() the shape of three-dimensional eights.
the shape of three-dimensional eights.
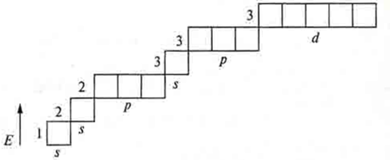
Element # 16: Sulfur, the charge of the nucleus of an atom is +16, and there is no charge in an atom of 16.
n = 3, there are three energy levels in an atom:
n = 1, the first energy level;
l = 0 — s-sublevel;
ml = 0, one orbital, is spherical.
1s; 1![]()
n = 2, the second energy level;
l = 0.1; two sublevels;
l = 0 — s-sublevel;
ml = 0 — one spherical orbital.
2s; 2![]()
l = 1; p-sublevel;
ml = +1, 0, -1; three orbitals, three-dimensional eights.
2p; 2![]()
n = 3, the third energy level:
l = 0, 1, 2 — three sublevels;
l = 0; s-sublevel;
ml = 0, one orbital, is spherical.
3s; 3![]()
l = 1, p-sublevel;
ml = +4, 0, -1; three orbitals; volume eights.
3P; 3![]()
l = 2, d-sublevel;
ml = +2, +1, 0, -1, -2 — five orbitals of more complex shapes.
3d; 3![]()
You can write it like this:
Characteristics of elements # 5. # 14 students perform at the blackboard (write briefly). Students of the class discuss the answers to questions § 2.
№ 1
An electron in an atom has no trajectory, i.e. we can only talk about the probability of finding it in the space around the nucleus.
№ 2
An electron cloud is a volume of space relative to the nucleus, in which all the mass and charge of e- are concentrated.
Orbital — an atomic orbital, the volume of space around the nucleus, in which about 90% of the electron density is concentrated.
№ 4
2s-the orbital has a large radius compared to 1s, and the larger the AO radius, the greater the energy: EAO1sEAO2s.
№ 5
The main quantum number determines the total energy of an electron at a given energy level. The period number corresponds to the number of energy levels in the atom of a chemical element.
№ 6
The electrons of the energy level differ from each other in their binding energy to the nucleus of the atom, resulting in sublevels. The period number corresponds to the number of sublevels at a given energy level. If bromine is located in the IVth period, then its atom has four energy levels, on the fourth energy level there are four sublevels; the charge of the nucleus is an atom of bromine +35, in the atom there are 35 electrons. We now need to find out how the electrons of an atom of a chemical element, possessing a certain amount of energy, which is characterized by quantum numbers, are distributed in the atom.
III. Learning new material
Plan of presentation
1. The principle of minimum energy, the Pauli exclusion principle. The rule of khunda and the consequence of it; rule Kleczkowska.
2. The allocation formula; the formula is fill.
3. fill — in Formulas-electronic formulas, graphic-elements of I — III periods.
4. filling Formulas (electronic and graphical) for elements of large periods.
5. Families of chemical elements.
6. Symmetry of an atom of a chemical element.
Electronic formula-a record of the structure of an atom by means of electronic levels, sublevels.
To properly fill the AO with electrons, you need to know the following: an electron occupies that energy level, that sublevel or that AO, which corresponds to the minimum energy reserve.
This is the principle of minimal energy.
Therefore, the energy level, that sublevel, that AO, which is closer to the core, is filled: 1s, 2s, 2P, ...
Pauli principle. There can't be two e-(electrons) in an atom that have all four quantum numbers characterized by the same values. The electrons must differ by at least one quantum number value. A corollary of this principle is that there can be no more than two electrons in one AO, characterized by different values of the spin quantum number.
Example: the electronic formula of an atom of a chemical element is Given.
1s2; n = 1; l = 0 one sub-layer, s;
ml = 0 one orbital, sphere;
![]()
electrons fill the s-Pro-level of the AO; the graphic configuration of the electronic formula is recorded.
ОрбитальWe draw the orbital as a cell, and the electron as a vector.
![]() — on the Pauli principle.
— on the Pauli principle.
 contrary to Pauli's principle.
contrary to Pauli's principle.
Правило Hund's Rule (Gunda). When distributing electrons in the sublevels p-, d -, and f -, it should be remembered that the total spin was maximal.
Example:
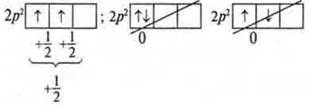
2p3-sublevel
Corollary of Hund's rule.
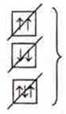
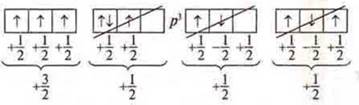
The electrons first occupy all the AO one by one, and then go to pair.
![]() —
unpaired electrons (one per AO).
—
unpaired electrons (one per AO).
![]() —
paired electrons (two per AO)
—
paired electrons (two per AO)
![]() —
one pair of paired e-s and two unpaired e-s .
—
one pair of paired e-s and two unpaired e-s .
However, in the atom there are AO with the same energy reserve, but located at different energy levels. How do I fill them out? We need to consider the same principle of minimal energy.
We apply the rule of Klechkovsky (1861): first, we write down that AO, that sublevel, that energy level, where the sum of quantum numbers n + l will be less in the case of equal values of n + l, that level, where n-less.
Example: Element K is located in period IV and opens it. However, in the third period, the AG p sub-level was only completed р у Аr.
What will be filled in first?
3d? Or 4s?
3d: the sum of n + l = 3 + 2 = 5.
4s: the sum of n + l = 4 + 0 = 4.
4 < 5 ⇒? first fill in with 4s, and then we think again: 4p? or 3d?
4P: the sum of n + l — 4 + 1 = 5;
3d: the sum of n + l = 3 + 2 = 5.
The value of the sums is the same, but according to the principle of minimal energy E at n = 3 is less than E at n = 4, so 3d and then 4P will be filled in.
So, it should be remembered that in an atom, each electron is located so that its energy is minimal, which corresponds to the greatest connection of it with the nucleus.
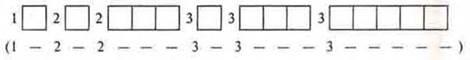
It should be known that there is a distribution formula where we can write down the energy levels and sublevels that open at the energy level:
![]()
However, taking into account all the rules and principles, there is a filling formula.
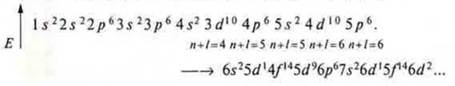
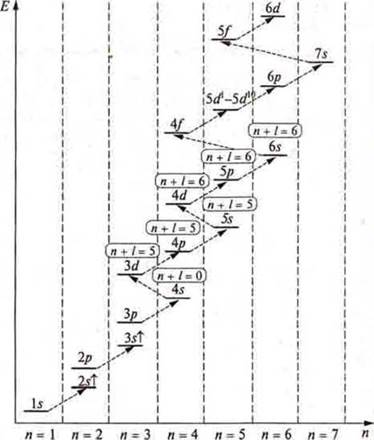
The location of sublevels by energy and the order in which they are filled with electrons (shown by arrows)
Let us consider the filling of atoms of elements of small periods with electrons.
Example:
a) the element hydrogen, H; the charge of the nucleus +1; electrons-1.
n = 1; l = 0; ml = 0 (one orbital)
s is the sublevel of ms= +1/2.
Electronic formula of hydrogen:
Graphic image of the electronic formula:
b) the element helium, Not; the charge of the nucleus - +2; 2e-- two electrons.
n = 1; l = 0; ml = 0; ms = ±1/2.
electronic formula 1s2.
graphic
image: ![]() paired
electrons.
paired
electrons.
For elements of the second period, according to the principle of minimum energy, the first energy level will first be filled, and then it will be filled according to the principle of minimum energy, Pauli's principle, Hund's rule — the second energy level.
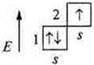
a) Li-lithium; +3; 3E-.
n = 2; 1s22s1.
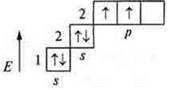
b) C-carbon; +6; 6E-; n = 2; 1s22s22p2.
Next, students work with the table. 2 textbooks on p. 16-17 and draw conclusions about the incomplete and completed energy level.
At the ne neon atom: +10; 10E-; 1s22s22P6.
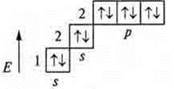
At the second energy level, the maximum amount of e-
n = 2n2; n = 8.
The energy level is complete.
The elements that have the s-sublevel filled in are called s-elements, and the p-sublevel is called p-elements.
The elements of the third period have three energy levels in the atom. The first and second energy levels are full.
1s22s22p6 is the ne neon structure, and at the third energy level, three sublevels of 3s3p3d open, because at n = 3, l = 0, 1, 2.
From sodium to magnesium is filled with 3s to 3s2. These are s-elements. From aluminum to argon, 3P to 3P6 is filled— these are p-elements. The third period ends with argon 1s22s22p63s23p6, whose external energy level has an octet of electrons, it is stable.
The element of the fourth period in an atom has four energy levels, since n = 4.
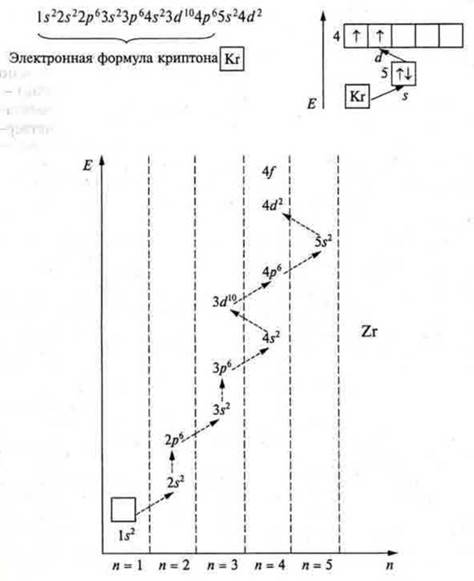
Under rule Kleczkowska have To and CA is filled in with 4s to 4s2-these are s-elements. Then, starting with Sc and ending with Zn, according to the same Klechkovsky ruleКлечковского, 3dto 3d10 (zinc) will be filled — these will be d-elements. The fourth period ends with p-elements, starting with Galium and ending with krypton, which at the fourth energy level, just like neon, argon, has an octet of electrons, a stable energy level.
For elements of the fifth period, fill in 5s → 4d → 5P, and for the sixth period, fill in 6s → 5d1→ 4f → 5d10→ 6P.
4f is a lanthanide;
f-elements of the seventh period — 7s → 6d1→ 5f → 6d10→ 7p.
5f is the actinides, the f-elements.
Thus, depending on which sublevel is filled in (s -, p-, d -, f -), elements are divided into families: s-elements, p-elements, d-elements, and f-elements; s - and p-elements are located in the main subgroups:
s-elements - in the main subgroups of I, and groups;
p-elements - in the main subgroups of groups III—VIII;
d-elements are arranged in side subgroups;
f-elements are two families-lanthanides and actinoids.
It should be remembered that for the correct addition of AO it is necessary to apply the principle of minimal energy, the Pauli and Hund principles, and the Kleczkowski rule.
However, there are some exceptions for 10 elements: Withu, Ag, Au, Cr, Md, Nb, Ru, Rh, Pd, Pt. In the atoms of these elements, there is a spontaneous transition of one electron (in Pt — two) from the s-sublevel of the external energy level to the d-sublevel of the external energy level. This phenomenon is called an electron dip or skip.
It is associated with the gain in energy, with the symmetry of the AO.
An atom is considered symmetrical if most of the electrons are either pairedor unpaired.
III. Homework assignment
§ 3 № 1, 2, 5, 6 (oral); Nos. 3, 4, 7-in writing.
IV. Consolidation
Task: Create an electronic formula and a graphic image of the electronic formula of element # 40.
Answer: zirconium, the charge of the nucleus is +40, there are 40 electrons in an atom, which are distributed over five energy levels, since n = 5. This is a d-element, since it is located in a side subgroup.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.