
Lesson-lecture on the topic " Valence capabilities of atoms of chemical elements. Oxidation degree»
The purpose of this lesson: to give the concept of "valence", "valence electrons", learn to identify the valence of the atom in the ground state; to consolidate the knowledge of the excited state of the atom and learn to identify its valence possibilities; to consolidate knowledge of the concept of "oxidation state" (S. O.), learn to identify S. O. V binary compounds and more complex structure; to be convinced of the essential differences between the concepts of "oxidation state" and "valence atom"; to give an idea of the atoms-the atoms of the donors and the acceptors.
Basic concepts: valence, ground and excited state of an atom, valence electrons, donor atom, acceptor atom, degree of oxidation.
Equipment: PSHA of D. I. Mendeleev table "Electronic structure of the atom".
Lesson progress
I. Organizational moment
The teacher informs students about the results of work in the lesson-seminar, considers typical mistakes in performing independent work, and
there are also homework assignments in some students ' workbooks. It is recommended to explain some of the comments to students individually. It is mandatory to note the positive work of students in their workbooks.
In the future, you should familiarize students with the topic of the lesson and the goals that will be set for them during the study of this issue. You should familiarize yourself with the plan of presentation of the educational material (it is desirable to write it either on the blackboard or on the codotransport).
II. Introductory conversation
Have you ever thought about this fact, why does a phosphorus atom form many compounds?
a) with hydrogen — PH3.
b) with oxygen-P2O5; P2O3;
chlorine atom:
a) with hydrogen-Hcl;
b) with oxygen — SL2O3; SL2O; SL2o7.
We will have to find out why this is possible.
III. Learning new material
Plan of presentation of the material
1. The ground state of the atom. Valence. Valence electrons on the example of Li, C, H, CL, MP atoms.
2. Valence capabilities of chemical element atoms, donor atom, acceptor atom.
3. the Excited state of the atoms of a chemical element.
4. The degree of oxidation. Definition of S. O. in connections;
minimum intermediate and maximum-S. O.
The concepts of SW and valency and their essential difference.
Atoms of chemical elements in the ground state at the external energy level can have paired and unpaired electrons. The electrons of these levels, and sometimes of earlier levels, can take part in the formation of chemical bonds.
Such electrons are called valence electrons. First of all, the valence of an atom is determined by the number of unpaired electrons involved in the formation of a chemical bond. Further, the teacher's explanation is accompanied by the compilation of a table.
In s - and p-elements, valence electrons are located at the s - and p-sublevels of the external energy level.
For d-elements - at the s-sublevel of the last energy level and d-sublevel of the previous energy level.
For f-elements — at the s-sublevel of the last energy level and d-sublevel of the penultimate energy level and f-sublevel, the third from the edge of the energy level.
A table is compiled together with the teacher.
Arrangement of valence electrons of elements of different families
|
The families of elements |
Sublevels where valence electrons are located |
Energy levels |
|
of s-elements |
s-sublevel of |
the outer energy level |
|
of R-elements |
s - and p-sublevels |
energy level of |
|
d-elements |
s-sublevel the d sublevel of |
outer energy level predvneshnem energy level |
|
of the f-elements |
s-sublevel d sublevel f sublevel of |
outer energy level predvneshnem energy level third from the edge of the energy level |
All valence electrons determine the basic properties of elements. Levels, sublevels, on which valence electrons are located, are called valence.
Students together with the teacher make electronic and electronic-graphic formulas of s-, p-, d -, f-elements in the basic state.
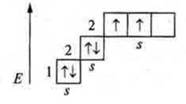
Lithium (Li): +3; 3E-, 1s22s1; s-element, atom has one unpaired s-electron, the valence of lithium — 1.
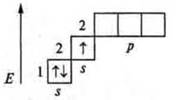
Phosphorus (P): +15; 15E-.
1s22s22p63s23p3; p-element.
Phosphorus has five valence electrons — two paired s-electrons and 3 unpaired p-electrons.
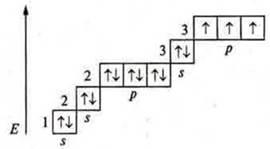
Manganese (MP): +25; 25E-.
1s22s22p63s23p64s23d5; d-element.
Manganese has seven valence electrons, two paired s-electrons, and five unpaired d-electrons.
However, it should be remembered that first of all, unpaired electrons of the last energy level enter into the reaction. Depending on the filling of the last energy level, chemical elements are divided into active, if there are unpaired electrons, and inactive, if there are paired — paired electrons. Based on the structure of the atom in the ground state, it is possible to determine the metallicity and non-metallicity of the element. If there are less than three electrons at the last energy level — it is a metal element; in our case, Li, MP are metal elements; if there are more than four electrons at the last energy level, it is a non-metallic element, in our case it is R. Therefore, all s-, d -, and f-elements are metal elements; and p-elements can be both metallic and non-metallic.
In PSE on the diagonal from B to At, all p-elements above the diagonal are non-metallic, below-metallic. According to the structure of the atom, elements that have three or four electrons at the external energy level are considered transition elements; many d-elements are metallic with transition properties.
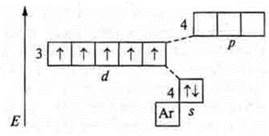
Thus, the valence of an element is primarily determined by the number of unpaired electrons of the external energy level. However: how can a carbon atom in the ground state C; +6; 6E-, 1s22s22p2, p -element, 2 unpaired electrons (hence, its valence is two) show a valence of four?
It is known that if there are free orbitals in the atom of an element, then in the case of available energy from outside, it is possible to vaporize paired electrons and transfer the electron to a free orbit. In this case, the atom is in an excited state. In the case of a carbon atom, there is a free orbital at the p-sublevel. In the excited state, there is a transition of one s-electron from the 2s-sublevel to the free orbital of the 2P-sublevel. The cost E is equal to 400 kJ/mol. It is compensated by the formation of two mol C-H bonds, and 720 kJ/mol is released. which exceeds the energy of carbon atom transfer to the excited state by 320 kJ/mol.
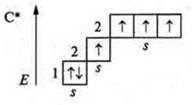
As a result, four unpaired carbons are formed in the carbon atom; the carbon valence is equal to four.
Question: What determines the valence potential of atoms?
Answer: the Valence capabilities of atoms are determined by the number of unpaired electrons in the main and excited state.
However, the valence capabilities of atoms can be determined by both the number of empty orbitals and the number of undelivered electron pairs.
The atom providing the lone pair is the atom-donor atom-acceptor free orbitals and gives their unshared pairs of electrons.
Example: Nitrogen (N): +7, 7E-.
1s22s22p3; p-element.
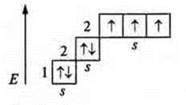
At the external energy level in the ground state: a pair of paired s-elements and three unpaired p-electrons, the valence by the number of unpaired electrons — three, as well as a nitrogen atom — a donor atom, provides an unpaired pair of s-electrons. In the ground state, the nitrogen atom exhibits a valence of four.
Let's make a General conclusion about the valence capabilities of atoms of chemical elements.
The valence capabilities of atoms are determined by:
1) the number of unpaired electrons in the main and excited state;
2) the presence of undelivered pairs of electrons, and being donor atoms;
3) the number of empty orbitals, and be acceptor atoms.
As a reinforcement, students perform a task: to determine the valence capabilities of atoms of a) phosphorus; b) chlorine in the main and in the excited state.
Phosphorus (P): +15; 15E-.
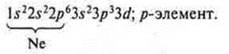
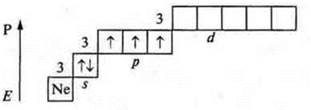
In the ground state a phosphorus atom can exhibit valence:
- three-because there are three unpaired p-electrons in an atom;
— four — because a phosphorus atom, a donor atom, can provide a pair of electrons to form a chemical bond.
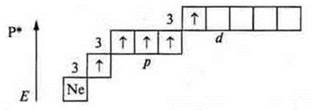
If the phosphorus atom is in an excited state, then the transition of the s-electron from the sublevel 3s to the sublevel 3d — to a free orbit is possible, as a result of which five unpaired electrons appear in the atom, which ensures the valence of the phosphorus atom — five.
Chlorine (CL), +17, 17E-;
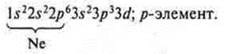
In the ground state, the valence is one (one unpaired p-electron), the chlorine atom is a donor and can provide pairs of paired electrons and exhibit in the ground state a valence equal to one, two, three, four.
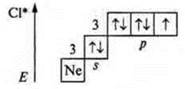
The first excited state is the transition of a p-electron (one to the 3d sublevel): three of the unpaired electron with a valence of three; atom-donor — valence — four, five.
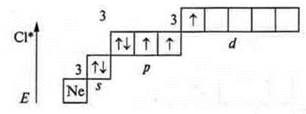
The second excited state is the transition of a p-electron (the next one from the p-sublevel to the 3d sublevel): five unpaired electrons — valence — five; atom-donor-valence-six.
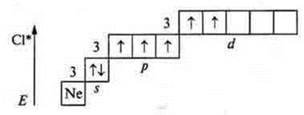
The third excited state is seven unpaired electrons; the valence is seven.
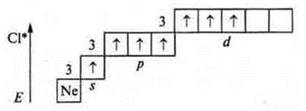
The maximum valence of atoms of chemical elements of period II is always four, since at the II energy level it is possible to open only four orbitals: one s-and three p -; for elements of period III, it is possible to open 9 orbitals — one s -; three p-and five d -. They can exhibit valency up to the maximum (9), but only 8 is known. (In the future, we will find out exactly which elements and in which compounds have this valence.)
As a rule, the valency is written with a Roman numeral for the element in the compound.
Example: ![]()
However, the elements in the compound are determined not only by the valence, but also by the degree of oxidation. Is this a single concept?
The valence of an atom is the number of common electron pairs that a given atom forms with other atoms in a given compound, taking into account its valence potential, which it has shown in a given compound.
Oxidation state (O. S.) — the standard charge, which becomes the atom in the compound in case of displacement of the electrons from the atom a positive charge and Vice versa, the displacement of the electrons to the atom with negative charge, if you count all the connections in the substance ion.
If in the compound the atom does not exhibit valence as a donor, then the degree of oxidation (C.O.) numerically coincides with the valence.
Example:

In the formation of the bond, nitrogen acted as a donor atom. S. O. can be minimal (min), maximum (Shah) and intermediate.
C. O.minis defined by the number of valence sites in the incomplete energy level for non-metallic elements. The carbon atom has a C. O.mjn= -4, because up to the stability of the external energy level of the carbon atom — up to 8 electrons — the number of valence places is four, the nitrogen atom has a C. O.min= -3; oxygen has a C. O.min= -2.
The maximum CP is determined by the number of electrons of the external energy level of s-and p-electrons (sum), as well as the sum of s - and d-electrons for d-elements.
Example: a manganese atom is a metal d-element. In metal elements, the SR corresponds to the number of electrons of the external energy level.
For metal elements s-or +1, +2: s-and p - +3, +4 for d-elements, MP 4s23d5is the minimum, for manganese +2; the maximum sum of s-and d-electrons is +7.
Intermediate CPUs are possible from 0, +1, +2 +3 +4, +5, +6, +7. however, they are considered stable 0, +2, +4, +6, +7.
Next, it is necessary to explain to students the definition of S. O. elements in compounds.
a) binary connections:
![]() —
determine the element more electronegative, to which the electrons are shifted,
and its minimum CP, in this case, it is sulfur; min = -2.
—
determine the element more electronegative, to which the electrons are shifted,
and its minimum CP, in this case, it is sulfur; min = -2.
The molecule is always electroneutral, therefore the product of the So-Called electronegative element on the number of atoms is equal in absolute value to the product of its electropositive element on the number of atoms.
2x = 6; x = 3. S. o. aluminum+3;
b) compounds of more complex structure:
![]() — put
the C. O. of known elements, in this case it is oxygen -2 and potassium +1;
unknown element-X.
— put
the C. O. of known elements, in this case it is oxygen -2 and potassium +1;
unknown element-X.
Next, in the same way as in binary compounds.
(+1) · 1 + x + (-2) · 4 = 0;
+ 1 + x-8 = 0; 1 + x = 8; x = +7.
The absolute value is true for x = +7.
1 + x = 8; x = 7; x = +7;
either according to the scheme:
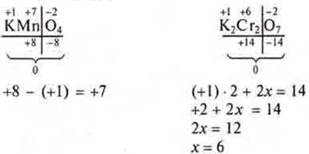
If a compound with a nonpolar covalent bond is a simple substance, then the CP is 0.
![]()
In organic compounds, the carbon Content is determined for each individual, taking into account the Carbon content of the elements associated with it.
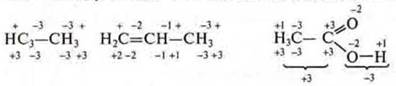
Carbon in all compounds is tetravalent, but the SR has different properties. This fact once again confirms that S. O. and valence are not the same concept.
II. Homework assignment
§ 4, № 5, 6, 7 (S); Nos. 1, 2, 3 (orally).
Some students may be asked to prepare messages for their next lesson on the topic:
1. Works of D. I. Mendeleev's scientific predecessors.
2. D. I. Mendeleev's discovery of the Periodic law.
3. Periodic law and structure of the atom. Modern wording of the law.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.