
Preparation of IBS by electronic balance method - CHEMICAL REACTIONS - LESSON PLANS for CHEMISTRY class 11 - lesson plans - lesson plans-author's lessons-lesson plan-lesson summary - chemistry
Objectives of the lesson: to give an idea of the method of electronic balance in the compilation of IIA; to improve the ability to reflect the essenceOBR by electronic balance method, fixing the concepts of oxidation, reduction processes: fix the concepts of "oxidizer", "reducing agent".
Basic concepts: algorithm, electronic balance scheme, oxidation, reduction processes, oxidizer, reducing agent.
Lesson progress
I. Checking students ' knowledge
1) Write down the OVR equations from the text of § 11, specify the type of OVR, classification type, oxidizer, reducing agent.
![]()
reaction![]() of
the reducing agent compound, since
of
the reducing agent compound, since
![]() increases
S. O. to
increases
S. O. to![]() so3;
so3;
![]() -
oxidizer, because
-
oxidizer, because![]() it
lowers the PH to
it
lowers the PH to![]() b
b ![]()
OVR intermolecular;
![]()
decomposition reaction:
KMPO4is an oxidizer, since![]() it
reduces the S. O. in compounds
it
reduces the S. O. in compounds![]() and
and ![]()
KMPO4is a reducing agent, since![]() в
в ![]() it
increases the S. O. in the compound
it
increases the S. O. in the compound![]() .
.
OVR is an intramolecular, and for manganese, it is also a disproportionation reaction;
![]()
substitution reaction:
![]() increases
CPR to
increases
CPR to![]() a
reducing agent;
a
reducing agent;
![]() in
the compound SG2O3 reduces the So — o to
in
the compound SG2O3 reduces the So — o to ![]() -oxidizer;
-oxidizer;
OVR — intermolecular.
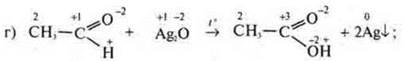
reaction of incomplete oxidation of an organic compound;
OVR intermolecular, because![]() in
aldehyde increases the S. O. to
in
aldehyde increases the S. O. to![]() in
acid, aldehyde-reducing agent.
in
acid, aldehyde-reducing agent.
![]() it
lowers
it
lowers![]() the
PH toAg 2O-oxidizer.
the
PH toAg 2O-oxidizer.
IDW are some of the reactions of compounds, decomposition, and substitution reactions.
The oxidizer-always lowers the PH.
The regenerator is always S. O. p. increases.
Question: As an oxidizer or reducing agent are the particles in the schemes? What processes are underway?
![]() —
increase of S. O. A0-reducing agent (V-l), oxidation process —
electron recoil;
—
increase of S. O. A0-reducing agent (V-l), oxidation process —
electron recoil;
![]() —
increase of CP In-3— reducing agent( V-l), oxidation process —
electron return;
—
increase of CP In-3— reducing agent( V-l), oxidation process —
electron return;
![]() —
reduction of CP D+6-oxidizer (o-l), the reduction process — electron
attachment.
—
reduction of CP D+6-oxidizer (o-l), the reduction process — electron
attachment.
![]() —
reduction of C. O. s. 0-oxidizer (o-l), the reduction process —
electron attachment.
—
reduction of C. O. s. 0-oxidizer (o-l), the reduction process —
electron attachment.
So, the processes:
Reduction (b-e) — joining of electrons by an atom or particle.
Oxidation (o-e) — the return of electrons by an atom or particle.
Then there is a transition to the study of new material.
II. Learning new material:
Plan of presentation:
1. Methods or techniques of preparation IA.
2. The method of electronic balance. Algorithm.
3. Some cases that should be remembered when compiling an IIA using the electronic balance method.
There are several techniques for compiling an IAB. We need to thoroughly understand and be able to apply the electronic balance method in the compilation of IIA. However, in the case of very complex OVR, when not just atoms or ions, but particles with a certain charge participate as an oxidizer and reducing agent, the electron-ion method of composing OVR is used. Students who will take the chemistry exam when entering the University should know this method. When composing an OVR using this method, knowledge of the OVR environment (acidic, alkaline, neutral) is mandatory.
In order to quickly compile an IAB, save time, especially when performing test tasks where only a short answer is required, there is a subscript method for compiling an IAB.
An algorithm for compiling IIA using the electronic balance method
1. Draw up a reaction diagram and determine the Percentage of elements in the initial and final products of the reaction:
![]()
2. To determine:
a) the element that promoted the S. O.;
b) the element that lowered the S. O.
3. Draw up the scheme of electronic balance, reasoning as follows.
![]()
Element A S. O. raised from 0 to +n, acted as a reducing agent, and itself was oxidized, because there is a process of electron recoil. Oxidation.
b) the Element In0C. O. has been lowered from 0 to -m, acted as an oxidizer, and itself has been restored, since the process of electron addition is underway — reduction:
![]()
Please note that some concepts are abbreviated:
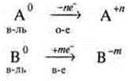
This is the beginning of the electronic balance scheme.
C) it is Necessary to balance the number of electrons between the oxidizer and the reducing agent. Find the smallest common factor and determine the coefficients before the oxidizer and reducing agent, before the final products of the reaction.
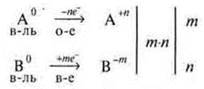 m·n —
the smallest common multiplier
m·n —
the smallest common multiplier
d) in the reaction equation, we write the coefficients, as a rule, at the beginning, before the reaction products in the right part of the equation (where there are elements with a change in the C. O.), and then compare the number of atom particles with the left part of the equation and put the coefficients before the substance of the initial reaction products.
It should be remembered! In IAB, the second-to-last one equalizes hydrogen, and the last one compares the amount of oxygen in the left and right sides of the equation.
If the oxygen atoms in the left and right sides of the equation do not match, check that they are correct:
a) definitions of CP and elements;
b) oxidation and reduction processes in the electronic balance scheme (the number of accepted and given (electrons);
C) determining coefficients:
![]() — OVR
equation with coefficients.
— OVR
equation with coefficients.
Let's take a concrete example:
![]()
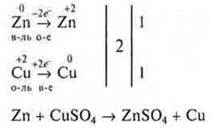
Coefficient 1-don't set it.
Compare the o atoms: there are four atoms in the left and right parts.
Special cases in the development of an IIA
1) If both reagents and the reaction product in the molecular state (O2;N2;Cl2) participate in the reaction, this is taken into account when drawing up the electronic balance scheme.
Example:
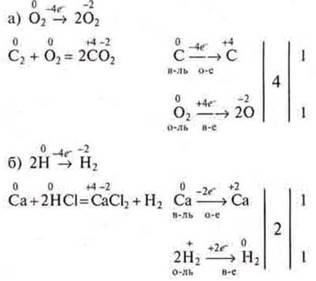
2) Sometimes we put the found coefficients before the original products in the left part of the equation.
In switching reaction equations, the coefficients are placed first in the left part of the equation.
Example:
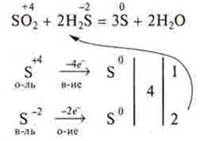
3) for substances in which the atoms of two, three, etc.elements are simultaneously oxidized and reduced, the calculation is carried out per particle molecule. For intramolecular redox reaction.
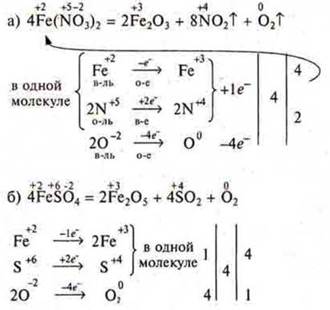
4) for substances in which the atoms of two elements are simultaneously oxidized (or reduced), the calculation is carried out for one molecule of the substance.
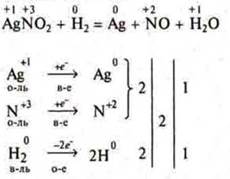
III. Pinning
Let's take a concrete example of other cases.
Example 1.
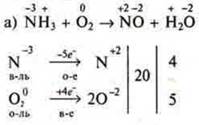
![]()
In this equation the right side the oxygen atoms are in two compounds, so the coefficient 5 set for oxygen in the left part of equation 4 and NH3at the left, and then hedged by the scheme.
Example 2.
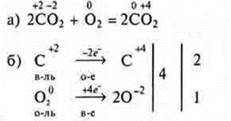
Example 3.
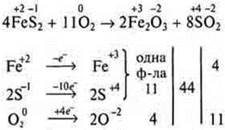
Example 4.

IV. Homework assignment
1. Summary.
2. Create an IIA using the electronic balance method:
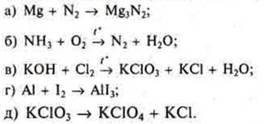
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.