
The dispersed system, a DISPERSED SYSTEM. SOLUTIONS. PROCESSES OCCURRING in SOLUTIONS - LESSON DEVELOPMENTS in CHEMISTRY grade 11 - lesson developments-lesson developments - author's lessons - lesson plan-lesson summary - chemistry
The purpose of the lesson: to give an idea of dispersed systems and their classification; to show the value of dispersed systems in nature and human life, the relative division of solutions into true and colloidal.
Basic concepts: dispersed system, coarse-dispersed system, fine-dispersed system; suspensions, emulsion, suspension, aerosol, colloidal system; Sol, gel, true solutions, Tyndall effect, dispersion medium, dispersed phase, pure substance, mixture, syneresis, micelle.
Equipment: household chemicals, aerosols; cosmetic, medical, food gels; ointments, pastes; cast iron, steel and products made from them; rock samples, minerals; water, vegetable oil (to demonstrate emulsions); gelatin, water (to demonstrate jellies); NaCl, benzene, Cologne, mineral water (to demonstrate the relative division of solutions into true and colloidal: Na2SiO3, HCl, FeCl3, NH3(R-ry); AlCl33(TV.); HCl, alcohol.
Lesson progress
I. Organizational moment
Lesson on learning new material. If the school conducts scientific work, then in the lesson you can pre-sew an essay, the topic of which is questions related to the use of dispersed systems in human life, for example, on the topic "ingredients of cosmetics And their impact on the living body." In another case, a lecture with elements of conversation and demonstration of experiments is possible.
II. Learning new material
Plan of presentation
1. The concept of a dispersed system. Dispersion medium, dispersed phase.
2. Classification of dispersed systems by features:
a) state of aggregation;
b) particle size of the dispersed phase and dispersion medium:
— coarse-dispersed systems: emulsions, suspensions( suspensions), aerosols (messages));
— fine-dispersed: colloidal solutions (sols, gels); true solutions (messages of students) - molecular, molecular-ionic, ionic;
C) the degree of interaction between the dispersed phase and the dispersion medium.
3. the Significance of various dispersed systems in the life of man and nature.
The state of a pure substance is described as follows: solid, liquid, or gaseous. However, absolutely pure thingsdo not exist in PRHerod. Even a small amount of impurities can significantly affect the properties of a substance — its melting point, boiling point, electricalо- and thermal conductivity, and reactivity. Obtaining pure substances is the most important task of modern chemistry: only a pure substance shows its individual properties qualitatively.
There are the following substance labels:
|
Marking |
Degree of cleanliness |
The content of impurities |
of the scope of |
|
"CH» |
Clean |
2 · 10-5and 1.0 · 10-5% |
In the industry |
|
"BDA» |
Clean for analysis |
1,0 · 10-5-0,4 · 10-5 |
For analysis of technical products |
|
" XCH» |
A chemically pure substance |
5,0 · 10-6-0,0 · 10-6 |
In scientific research and laboratory work |
|
" osch» |
Especially a clean substance |
1,0 · 10-10-1,0 · 10-14 |
In electronics, semiconductors |
However, in nature, and in human life, there are not individual substances, but their systems. The most important of them are dispersed, i.e. heterogeneous systems-consisting of two or more phases with a strongly developed interface between them. Phase— чаpart of the system separated from other parts by the interface surface.
One of the phases is divided into small particles and evenly distributed in the volume of another substance — it is a dispersed phase, and the other — a solid phase — a dispersion medium.
Depending on the combination of the aggregate state of the dispersed phase and the dispersion medium, 9 types of such systems can be distinguished. Students should pay attention to the table No. 9, page 66 of the textbook.
Students make a table in their notebooks:
Classification of dispersed systems
|
Dispersion medium |
Dispersed phase |
||
|
gas |
liquid |
solid |
|
|
Gas |
Air, natural gas |
Aerosol, fog, associated petroleum gas |
Aerosol (smog), smoke, dust |
|
Liquid |
Fizzy drinks, foams, gas emulsions |
Emulsions (blood plasma, lymph, digestive juice, cytoplasm) |
Construction solutions, suspensions, sols (jelly, mud, glue, silt) |
|
Solid V-VA |
Powders, porous body, soil, textile fabrics, snow |
layer Medical and cosmetic products (ointment, mascara, lipstick), wet soil |
Minerals, alloys, rocks, colored glass |
Classification of dispersed systems according to particle size
|
Dispersed system |
Particle size, m |
|
1) Fine particle system a) True solutions b) Colloidal systems (Sol, gel) |
≤ 10-9 ≥ 10-9≤ 10-7 |
|
2) coarse-Dispersed system (emulsions, suspensions) |
≥ 10-7 |
Dispersed systems are classified according to the degree of interaction of the dispersed phase with the dispersion medium:
a) the interaction is weak: if the medium is water - based, the system is hydrophobic-silver halides; the system is lyophilized, if the medium is not water-based.
b) interaction is strong: if the medium is water-hydrophilic systems, they spontaneously form, have a high dispersion. If the medium is not water — based, then freeze-dried systems are thermodynamically stable and do not collapse over time.
The teacher shows an example of the system: proteins, rubbers. During the teacher's story, students examine samples of cast iron and steel.
For dispersed systems, the sign of heterogeneity is important. For example, iron alloys-cast iron and steel-differ in this way. The percentage of carbon in them is different. In cast iron more than 0.02 %, and in steel less than 0.02 %.
The excess carbon in the cast iron becomes a solid solution is released in the form of plates of graphite. Because of this, cast iron is brittle, unlike steel: steel is a solid, homogeneous solution of carbon in iron. Steel can be rolled, forged, stamped, and dragged. On the cast iron fracture, you can notice the gray color of graphite. When solidified, cast iron does not contract like most metals, but expands like water and ice. Cast iron is used only for casting. Thus, cast iron is a dispersed heterogeneous system.
Steel is a dispersed homogeneous system. It is a solid, true solution.
The teacher demonstrates some of the dispersed system:
coarse-grained: particle size ≥ 100 nm. These are opaque systems, particles are visible to the naked eye, are settled, and liquids have a visible interface;
a) emulsions: syntomycin emulsion-medicine; milk; lymph (natural), water-based emulsion paint, bituminous material, building emulsions.
Polymers are produced on the basis of emulsions: rubber, polystyrene, polyvinyl, and acetate.
b) suspensions: the dispersed phase is solid, insoluble in the dispersion medium it is liquid.
Example: "lime milk" - Froma(HE)2and N2O — building mortar; river and sea silt; chalk and N2O (show).
Suspensions are often called suspensions.
Coarse-dispersed systems of a very high dispersed phase are called pastes: toothpastes (demonstration — pastes, lipsticks, mascara).
Colloidal systems are classified as fine-dispersed and have a particle size of 100 to 1 nm. A colloidal particle is called a micelle. It has a rather complex structure, consisting of a particle of granules and a diffuse layer. Pellets can have both a positive and negative charge. This depends on the excess of one of the reagents. In the case of formation of a colloidal solution of AgNO3and NaCl, with an excess of Agno3, the granule has a positive charge, with an excess of NaCl, the granule is negatively charged.
Then there is a message from the students (or a teacher's story).
Colloidal systems are well distributed in nature. Soil, clay, natural water, air, clouds, dust, smoke, many minerals and even precious stones are all colloidal systems. Colloidal systems are of great importance in biology and medicine. All food products-bread, milk, butter-are colloidal systems.
From a chemical point of view, the body as a whole is a complex set of many colloidal systems, including liquid colloids and gels. Blood, plasma, lymph, and cerebrospinal fluid are systems in which proteins, cholesterol, glycogen, and other substances are in a colloidal state. And why does nature prefer colloidal States? In the colloidal state, the substance has a large interface between the phases, and this contributes to the better flow of the most important life process-metabolism.
For example: if you take a cube of matter with an edge of 10-2m, its surface is 6 · 10-4m2. If you break up the substance to the size of a colloidal particle 10-7-10-9m, then the total area of 1 m3of the substance will already be hundreds and thousands of square meters. And the speed of reactions, as we know, depends on the contact surface.
Colloidal systems are divided into sols and gels.
Sols — colloidal solutions isolated from each other by the particle phase and the dispersion medium.
Example: silicic acid, soluble glass Na2Sio33
Demonstration of an experiment:
but) ![]()
b) hydrolysisofAlCl3 in hot water gives Sol.
Gels are colloidal systems with contacting particles of phase and dispersion medium. These are gelatinous bodies. Particles of the dispersed phase are connected to each other in a loose spatial grid, in the cells of which the dispersion phase (medium) is contained. This system is devoid of fluidity.
Further examples of gels are discussed on the codotransport.
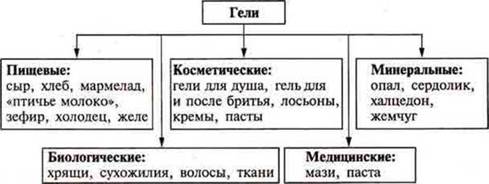
Demonstration: gelatin + H2O, starch paste.
For gels, the reaction of syneresis — spontaneous release of liquid is characteristic. The gel structure is tightened, the volume is reduced. The phenomenon of syneresis determines the shelf life of a product: food, cosmetic, or medical. Blood clotting is also cigeretes. There is a transformation of a soluble blood protein-fibrinogen - into an insoluble one-fibrin. This is a protective reaction of the body.
Sols can be subjected to coagulation. This is the combination of colloidal particles in larger aggregates and their precipitation under the influence of temperature, changes in concentration, mechanical action, irradiation, the addition of an electrolyte, the formation of a gel.
Externally, colloidal solutions are translucent and transparent solutions. They can not be distinguished from fine-dispersed systems — true solutions, where the particle size of the phase is less than 1 nm. Colloidal solutions are characterized by the Tyndall effect.
When passing a beam of light through a colloidal solution, large particles reflect light from their surface, and a glowing cone is visible in the vessel. Such a cone is not observed when passing a beam of light through a true solution, a finely dispersed system.
As a rule, colloidal solutions are homogeneous systems consisting of two or more substances of the same phase. One substance is distributed in another in the form of atoms, molecules, and ions. The solvent, dispersion medium, and aggregate state do not change during the formation of a dispersed system.
Example: solvent-dispersion medium water; in aqueous solutions of salts, acids, alkalis, sugar, carbon dioxide.
Air is a solution of oxygen, nitrogen, noble gases, and carbon dioxide.
Table vinegar 9 % - water is a solvent.
Vinegar essence 80% of the solvent acetic acid.
Solutions are divided into:
- molecular — water solutions of non-electrolytes (alcohol solution of iodine, solutions of sucrose, glucose);
- molecular-ionic-solutions of weak electrolytes (nitric acid, carbonic acid, ammonia water);
— ionic solutions — solutions of electrolytes.
It should be considered that dissolution is a physical and chemical process, since along with the formation of an ordinary mechanical mixture of substances, there is also a process of interaction of solute particles with solvents. D. I. Mendeleev came to such conclusions in 1887. Precise determination of a solution: a solution is a homogeneous system consisting of particles of a solute, a solvent, and the products of their interaction.
Experiment.

The copper (II) cation is being hydrated by water molecules. Hydrated ions give the solution a blue color, and heat is released — a sign of a chemical reaction.
II. Summary and conclusions
So, in this lesson, we have studied in more depth the classification of dispersed systems, their importance in nature and human life.
However, it should be noted that there is no sharp boundary between the types of dispersed systems. Consider the classification relative.
Depending on the conditions, one system may switch to another.
Example: blood is a colloidal solution and can become a coarse-dispersed system. FeCl3is a fine-dispersed system, but when heated, a colloidal solution is formed.
III. Homework assignment:
§ 8. Notes in a notebook. Definition. Questions § 8 verbally.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.