
Specific properties of inorganic and organic acids-SUBSTANCES AND their PROPERTIES - LESSON DEVELOPMENTS in CHEMISTRY 11 class - lesson developments-lesson developments-author's lessons-plan-lesson summary - chemistry
The purpose of the lesson: to consolidate knowledge of the General chemical properties of acids; to give an idea of the specific properties of inorganic and organic acids; to teach you how to correctly create reaction equations that confirm the special properties of inorganic and organic acids.
Basic concepts: the oxidizer, the reducing agent, the oxidation-reduction reaction.
Equipment: H2SO4(K), HNO33(K) (p), Cu, alcohol lamp, matches, test tubes, holder, CH3COOP, NSOOP, Ag2O (NH · H2O).
Lesson progress
I. Organizational moment
Setting both the goals and objectives of the lesson.
II. Independent work
|
Option I |
Option II |
|
1. Based on the classification of acids give the characteristic |
|
|
a) H2CO3; b) HI |
a) HF; b) H2SiO3 |
|
2. Draw up reaction equations that confirm the properties |
|
|
of HNO3 |
UNSW acid |
|
with substances: |
|
|
Al2O3; With aas(HE)2 |
CA, Na2CO3 |
III. Checking that homework has been completed correctly
At the time of performing independent work, the teacher individually checks the completion of homework.
§ 20 № 1
The stronger the acid as an electrolyte, the higher the a — degree of dissociation.
![]()
HNO2is a weaker acid, because C. O.N+ 3 is less than C. O.Nin HNO3. The greater the PH of the element, the stronger the acidic properties:
![]()
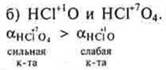 for
the reason of the different S. O.Cl.
for
the reason of the different S. O.Cl.
![]() because
the C. O.Sin H2SO4is greater than the C. O.sin
H2SO3.
because
the C. O.Sin H2SO4is greater than the C. O.sin
H2SO3.
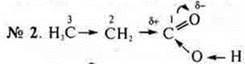
 it
affects the carbon-hydrogen bond in the pachannel, especially the bond of
carbon # 2 and hydrogen atoms, which gives an easy замещаемостьsubstitution
of hydrogen atoms for atoms, for example, halogen:
it
affects the carbon-hydrogen bond in the pachannel, especially the bond of
carbon # 2 and hydrogen atoms, which gives an easy замещаемостьsubstitution
of hydrogen atoms for atoms, for example, halogen:
δ+ of the carbon atom # 1 is quenched by the electron density of the radical, which causes a decrease in the displacement of the electron density from the group-OH, the dissociation of the acid decreases;
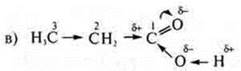
the π-bond between the carbon atom # 1 and the oxygen atom is shifted towards oxygen, where the electron density increases δ -, due to the displacement of the π -bond, the carbon atom # 1 has a lack of electron density, acquires δ+, which is partially extinguished by the electron density of the radical H3S—CH2—.
The oxygen atom of the group-OH, having a large electron density, shifts it towards carbon # 1, where δ+, which leads to the displacement of the General bond between oxygen and hydrogen of the group —O h in the direction of oxygen, making the hydrogen of the group —O h mobile. It's acid.
![]()

IV. Learning new material
Plan of presentation
1. Specific properties of H2SO4(K) and HNO33(K), (p).
2. Specific properties of some organic acids.
3. Performing exercises and calculating tasks of the topic.
Some representatives of inorganic acids have strong oxidizing properties to metals, non-metals, and complex compounds. This is caused by the fact that in sulfuric acid2SO4sulfur is in the maximum C. O., in nitric acid HNO3nitrogen is in the maximum C. o. +5. It is necessary to draw the attention of students to table 18 p. 250 of the textbook. This table should be used when writing reactions of nitric (p) and (K), sulfuric (K) acids with metals, non-metals, and complex substances.
According to the electrochemical series of metal stresses, we will divide all metals into three groups: from Li to Al — very active metals, from Al to H2-metals of medium activity, from H2 to Au — low-activity metals.
Together with the students, the teacher makes diagrams that more clearly represent the oxidative properties of acids. These diagrams show the IIA products.
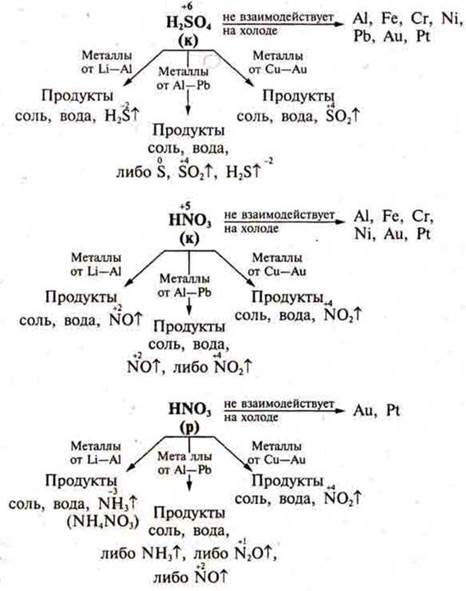
When drawing up these schemes, focus the attention of students on the change in the Concentration Of nitrogen and sulfur depending on the activity of the metal interacting with it.
It should be noted that the nonmetal is oxidized to an oxide or acid. When interacting with H2SO4(k), oxides of oxidizable nonmetals are formed, and if phosphorus is oxidized, then phosphoric acid, sulfur is reduced to sulfur oxide (IV).
With HNO3(k), nitrogen is always reduced to nitric oxide (IV), and nonmetals to oxides or acids.
With HNO3(p), nitrogen is reduced to nitric oxide (II), and nonmetals to oxides or acids.
Experiment: Cu is a low-activity metal.
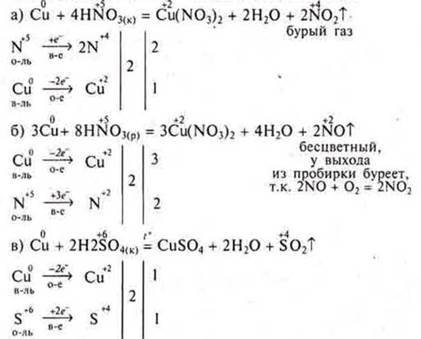
Oxidation of non-metals:
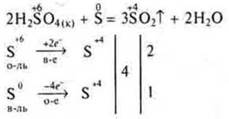
Oxidation of complex substances:
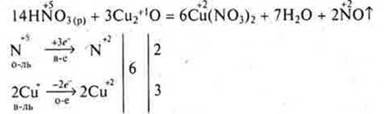
Inorganic acids interact with organic substances: reactions of nitration, sulfonation; nitration — the interaction of organic matter with nitric acid. Example: nitration of benzene, nitration of cellulose. The nitration of cellulose are formed dand- and trinitrocellulose — esters, it is very necessary substances for the production of smokeless gunpowder.
Specific properties of organic acids
Interaction with alcohols with the formation of esters.
Experiment:

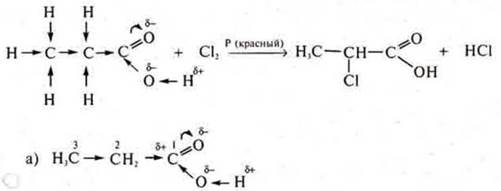
Organic acids enter into radical substitution reactions.
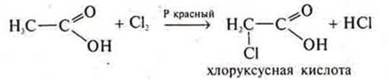
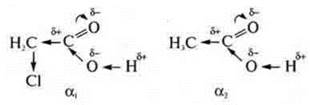
The introduction of an acid molecule of a halogen atom into a radical increases the degree of dissociation by 100 times.
Cl is the most electronegative element in the compound and will mix the electron density from carbon # 2 in its direction, resulting in a decrease in the electron density of the carbon # 1 atom, it acquires an even greater δ+ than in acetic acid. Oxygen atom in the group-OH with greater force will mix its electronic density in the direction of carbon # 2, thus making a very mobile hydrogen atom.
Some organic acids have dual properties.
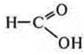 —
methane (formic) acid-aldehydeacid . It gives a "silver mirror" reaction,
acts as a reducing agent.
—
methane (formic) acid-aldehydeacid . It gives a "silver mirror" reaction,
acts as a reducing agent.
Experiment:
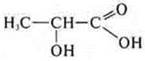 -
lactic acid, also has dual properties — it is an alcohol acid.
-
lactic acid, also has dual properties — it is an alcohol acid.
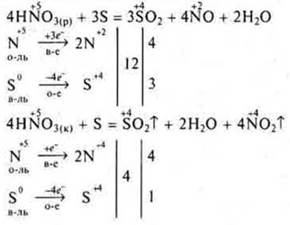
Some acids exhibit reducing properties.
Example:
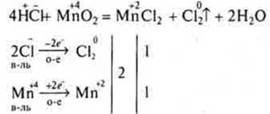
This chemical reaction makes it possible to produce SL2in the laboratory.
V. Generalizations and conclusions
Inorganic and organic acids have specific properties. Depending on the detachments with which they interact, and the conditions for the flow of reactions, they can exhibit redoxproperties. Many acids, especially organic acids, can not only have the properties of several classes of organic substances, but also exhibit redoxproperties.
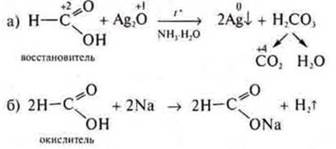
Example:
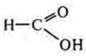 -
reducing agent interacting with Ag2O(NH3· H2O);
-
reducing agent interacting with Ag2O(NH3· H2O);
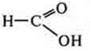 -
oxidizer, interacting with metals.
-
oxidizer, interacting with metals.
VI. Homework assignment:
§ 20 # 3, 5, 6; task # 19
VII. Pinning
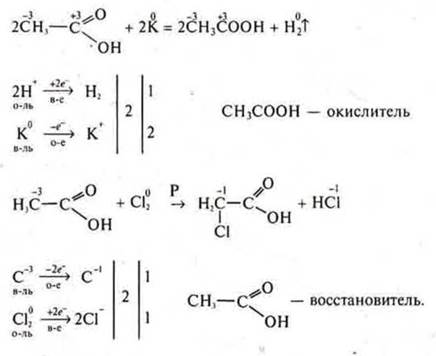
Consider окислительноthe redox properties of acetic acid:
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.