Презентация по аналитической химии на тему Метод титрования. данная лекция является вводной. В лекции раскрывается определения процесса титрования и метода титрования. Применение закона эквивалентов, расчет концентрации титруемого раствора с неизвестной концентрацией. Процесс приготовления титрованных растворов - стандартных и стандартизированных. В конце лекции дается викторина на знание основных определения и понятий данной лекции.
Analytical Chemistry
Titrimetry
Aim: To understand how to use titrimetry to
determine the concentration of different solutions
with unknown concentration
Lector: Nazira Mukhanbetova
master of chemistry
Contents:
1) To define the method of titrimetry and the
process of titration
2) Titration apparatus
3) Solutions for titration
4) How do we know when a titration reaction
is complete?
5) Types of titration
6) Application of titrimetry in food engineering
VOCABULARY
•Titrimetry – Титриметрия (метод)
•Standard Solution (titrant) – Титрант
•Analyte – Аналит
•Titration – Титрование (процесс)
•Equivalence Point – Эквивалентная точка
титрования
•End Point – Конечная точка титрования
•Burette – Бюретка
Titrimetry is an analytical method of
determining the concentration of a solution.
Titration
is a process where slow
addition of one solution of a known
concentration
to a
precisely measured volume of another
solution of unknown concentration (called
an analyte) and observing through some
is
means when
reached.
the end of
(called a
titrant)
reaction
Advantages of titration:
•Classical, wellknown analytical technique
•Fast
•Very accurate and precise technique
•High degree of automation
•Good price/performance ratio compared to more
sophisticated techniques
•It can be used by lowskilled and trained operators
•No need for highly specialized chemical knowledge
Major requirements for a titration reaction:
•Large Keq reaction goes to completion
•Fast speed; productivity
During a titration you have two solutions: the analyte
and the titrant.
•The analyte is the "unknown" solution for which you
would like to know either the concentration or the
equilibrium constant.
•The titrant (standard solution)
the "known"
solution which has a precise and accurate concentration.
is
to
During titration the titrant
is added to the analyte in
order
the
and
equivalence
the
determine
concentration
the
analyte.
achieve
point
of
Calculating concentrations
moles-equivalents solute (mol-eq)
Normality =
volume of solution
Titre =
(dm3)
grams of solute (mol)
volume of solution
(cm3)
The equation for concentration can be put into a
formula:
N
=
m
Eq*
V
m
V
T
=
Making Standard
Solutions
How do we know when a titration reaction
is complete?
The equivalence point: the point when
reacting. At
the
equivalence point law equivalence is performed:
reactants are done
V
titrant
V
analyte
N
N
analyte
titrant
N
analyte
V
titrant
V
N
titrant
analyte
The end point: the moment when the
titration is stopped. It is indicated by some
form of indicator which varies depending on
what type of titration being done.
Calculating Concentration of
V
titrant
V
analyte
N
N
analyte
titrant
Analyte
N
analyte
N
titrant
V
titrant
V
analyte
N
analyte
T
analyte
solute
)
(
Eq
1000
m
solute
total
VT
m
solute
m
total
W
,%
%100
Indication ways of point of equivalence
• Visual indication: subjectively observed change in the
appearance of titration solution, such as change of colour,
formation of precipitate, and fluorescence. The most often
employed is colour change of a suitable organic dye, an
indicator, added to titration solution. The change of colour
occurs just at the moment of when the point of equivalence is
achieved.
• Instrumental indication: some physical quantities of
titration solution are measured by instruments (for example
conductivity of the solution, pH etc.) in dependence on the
volume of added titration reagent – again, we get the titration
curve. Quantity is chosen so that the essential change in the
titration curve (e.g. a turning point, break) occurs in the point
is
titration
of equivalence. Consumption of
determined from a certain point of this change.
reagent
Indicators are substances that react either with
the determined substance or with surplus titration
reagent. The forms of indicator before and after the
reaction have different colours. One form transforms
to the other form in the point of equivalence.
Indicators are usually substances of the same
character as titration reagent or titrated substance.
There are many various indicators according to
character of titration.
Acidbase indicators
Metallochromic indicators of complexometric
titrations
Indicators of precipitation titrations
Indicators of redox reactions
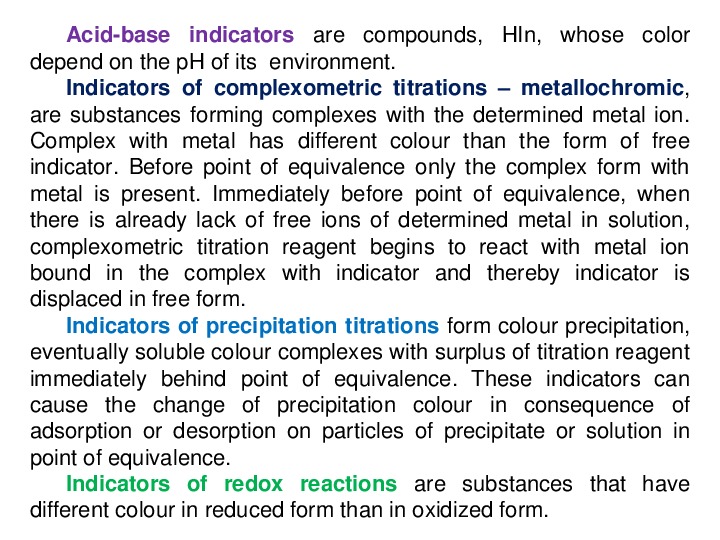
Acidbase
indicators are compounds, HIn, whose color
depend on the pH of its environment.
Indicators of complexometric titrations – metallochromic,
are substances forming complexes with the determined metal ion.
Complex with metal has different colour than the form of free
indicator. Before point of equivalence only the complex form with
metal is present. Immediately before point of equivalence, when
there is already lack of free ions of determined metal in solution,
complexometric titration reagent begins to react with metal ion
bound in the complex with indicator and thereby indicator is
displaced in free form.
Indicators of precipitation titrations form colour precipitation,
eventually soluble colour complexes with surplus of titration reagent
immediately behind point of equivalence. These indicators can
cause the change of precipitation colour in consequence of
adsorption or desorption on particles of precipitate or solution in
point of equivalence.
Indicators of redox reactions are substances that have
different colour in reduced form than in oxidized form.
What change will you observe in the solution
when the endpoint is reached?
At endpoint:
• moles of standard solution = moles of analyte
Start
Endpo
int
Past
Endpoint
Keep in mind
Equivalence point & End
point are not necessarily
equal
An endpoint is indicated by some form
of indicator at the end of a titration.
An equivalence point is when the moles
of a standard solution (titrant) equal the
solution of unknown
moles of
concentration (analyte).
a
Titration can be performed as:
Direct titration, where titration reagent is added
directly to solution of determined substance until the
point when substance amounts of both reagents are
equivalent.
Indirect
titration, where surplus of
titration
reagent
the solution of determined
substance first, and subsequently the formed product
is titrated.
is added
to
is added
Back titration, where an exact volume of titration
reagent
the solution of
determined substance, quantitative reaction runs over
and then the remaining surplus of titration reagent is
titrated by another titration reagent.
in excess
to
TYPES OF TITRATIONS
Acidbase titrations, in which an
acidic or basic titrant reacts with an
analyte that is a base or an acid.
Complexometric titrations involving
a metalligand complexation reaction.
Precipitation titrations, in which the
form a
titrant react
to
analyte and
precipitate.
Redox titrations, where the titrant is
an oxidizing or reducing agent.
fruits and juices
water
APPLICATIONS
OF TITRIMETRY
IN FOOD
ENGINEERING
drinks
meat products
SUMMARY
•“Titrimetry” – determination of analyte by reaction with
measured amount of standard reagent.
•“Standard Solution”
(titrant) –
reagent of known
concentration
•“Analyte” – reagent of unknown concentration.
•“Titration” – process where slow addition of titrant to analyte
solution from a volumetric vessel (buret).
•“Equivalence Point” – reached when amount of added
titrant is chemically equivalent to amount of analyte present in
the sample.
•“End Point” – the occurrence of an observable physical
change indicating that the equivalence point is reached.
•“Burette” – a graduated glass tube with a tap at one end, for
delivering known volumes of a liquid, especially in titrations.
QUIZ ME
1. Experimental method of determining
the concentration of an analyte is called:
Titre
Titrimetry
Titration
NEXT QUESTION
NEXT QUESTION
QUIZ ME
2. What is it Titration?
The process of diluting solution to the required
concentration
The process of slow addition of a titrant to a
precisely measured volume of analyte
The method of calculating solution concentration
NEXT QUESTION
NEXT QUESTION
QUIZ ME
3. For titration of 10 ml of NaOH solution 8
ml of hydrochloric acid (HCl) was used, of
concentration exactly c = 0.1 mol/l. What
will be the normality of titrated NaOH
solution? ?
0.8 mol/l
0.01 mol/l
0.08 mol/l
NEXT
3. For titration of 10 ml of NaOH solution 8 ml of hydrochloric acid (HCl) was used, of concentration exactly c = 0.1 mol/l. What will be the normality of titrated NaOH solution? ?NEXT
balance
ASSOCIATION CHART
Sample bottle
pallets
pipets
funnel
TITRATION
flasks
Graduated flask
cylinder
burette
SUMMARY
•“Titrimetry” – determination of analyte by reaction with
measured amount of standard reagent.
•“Standard Solution”
(titrant) –
reagent of known
concentration
•“Analyte” – reagent of unknown concentration.
•“Titration” – slow addition of titrant to analyte solution from a
volumetric vessel (buret).
•“Equivalence Point” – reached when amount of added
titrant is chemically equivalent to amount of analyte present in
the sample.
•“End Point” – the occurrence of an observable physical
change indicating that the equivalence point is reached.
•“Burette” – a graduated glass tube with a tap at one end, for
delivering known volumes of a liquid, especially in titrations.
REFERENCES
http://www.metrohmusa.com/Products/Titration/FoodTitration.html
http://ca.mt.com/ca/en/home/applications/Application_Browse_Laboratory_Analytics/Applicati
on_fam_browse_main.tabs.applications.html
http://www.ehow.com/list_5772040_titrationusedindustry_.html
http://www.pprc.org/research/rapidresDocs/RR_Salt_Content.pdf
http://www.labmanager.com/whitepapersandapplicationnotes/2011/05/metrohmusainc
analysisofsodiuminfoodstuffsbythermometrictitration?fw1pk=2#.VBv51pTOJ7w
http://www.aquaculture.ugent.be/Education/coursematerial/online
%20courses/ATA/analysis/mine_ana.htm
http://www.machinerylubrication.com/Read/984/analyzingwateroil
http://www.ehow.com/about_5406434_purposetitration.html
http://web.vscht.cz/~kohoutkj/ENG/LAPP_Detn_mineral_elements_v5.pdf
http://www.ehow.com/list_5772040_titrationusedindustry_.html
