
Laboratory work №3
Topic: Saturated hydrocarbons
The purpose of the work: Is to master the extraction of hydrocarbons by laboratory means and study their chemical properties.
Necessary equipment: sodium, potassium acetate, Natron trace, bromine water (light yellow), potassium permanganate ( liquefied solution).
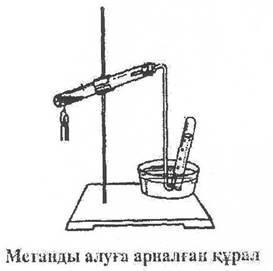
Course of work: It is advisable to take methane and ethylene in a test tube fixed with a cork with a short gas-conducting tube. At the end, we continue a 10x12 CM rubber tube with glass at the end. In a dry test tube, a mixture of 1 part of any anhydrous acetate and a part of natron Trace is placed, which is well pressed in a few grams, kilograms. We connect the gas-conducting tubes and fix the test tube in the horizontal direction. The mixture is first gradually, and then strongly heated. By placing the gas-conducting tube in separate test tubes containing bromine water and potassium permanganate, we will learn the reactions of bromine and oxidation by looking at the colors. Without stopping heating, we collect methane in a test tube filled with water in a tray with water. We remove the gas-conducting tube from the water and stop heating. We cover the test tube with our finger and bring it to the flame. Methane burns, giving a blue flame. If we put a porcelain plate on a hot methane flame, dark spots of soot will not appear.
Control questions:
1. Does methane dissolve in water?
2.Write down the equation of the methane combustion reaction.
3. Write methane formation reactions.
4. What changes occur when methane passes through the solution КМn04?
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.