
Laboratory work
Objective: experimentally determine the electron charge.
Instruments and materials: a cuvette with electrodes, a multimeter, a rheostat-potentiometer, an adapter, a scale, a vessel with a solution of blue vitriol, a clock, connecting wires.
Work instructions:
1. Suspend the carbon electrode from the two electrodes (carbon and copper) with the greatest possible accuracy.
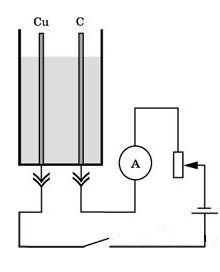
2. Place the carbon and copper electrodes on the pins of the cuvette, pour the solution of copper sulfate into the cuvette.
3. Assemble the electrical circuit as shown in Figure 46, while connecting the carbon electrode to the “-” minus of the current source.
4. Note the time and close the circuit. With a rheostat, set certain amperage and keep it constant throughout the experiment.
5. After the specified time (15-20 min), open the circuit, remove the carbon electrode, rinse it in water and dry it over the electric heater. Weigh this electrode again and calculate the electron charge by the formula.
Материалы на данной страницы взяты из открытых источников либо размещены пользователем в соответствии с договором-офертой сайта. Вы можете сообщить о нарушении.